Satisfactory surgical outcome of T2 gastric cancer after modified D2 lymphadenectomy
Introduction
In Japan, treatment guidelines for gastric cancer (GC) have been issued, and a standard therapeutic strategy for GC by stage has been established. Gastrectomy with D2 lymph node dissection has been increasingly regarded as the standard surgical procedure for advanced GC patients (1,2). Theoretically, if the dissected lymph node is rarely invaded, there is no need to remove it. The rate of lymph node metastasis was closely related to depth of invasion (3,4). In T1 stage GC, the lymph node metastasis rate was relatively low and D1 or D1 + lymphadenectomy was enough for such patients (1,5,6). For tumors with serosa invasion, more extended lymph node dissection, such as D2 or D2+ was required as the high incidence of lymph node metastasis (7-13). As for T2 stage GC, the 7th Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) TNM classification system only referred to tumor extending into the muscularis propria (14). Tumor invading to subserosa which was also included in T2 stage in the 6th UICC/AJCC TNM classification system was defined as T3 stage. The rate of lymph node metastasis was significantly lower in T2 stage GC than in T3/4 stage GC. Can the modified lymphadenectomy which was less extensive than D2 but more extensive than D1, such as D1 lymphadenectomy with dissection along the left gastric, common hepatic and celiac arteries, bring any benefits to patients with T2 stage GC? Controversy still exists. Until now, few studies particularly focused on clinicopathological characteristics and prognosis of T2 stage GC in the 7th UICC stage system (3,4,15-18).
In the present study, we compared the clinicopathological characteristics and surgical outcomes of T2 stage GC patients undergoing different extents of lymphadenectomy. Our ultimate aim was to identify the optimal treatment for T2 stage GC patients.
Materials and methods
Patients
The Ethics Committee of Tianjin Medical University Cancer Institute & Hospital has reviewed and approved this study. A total of 1,695 patients with GC who underwent surgical resection at Tianjin Medical University Cancer Institute & Hospital between January 2003 and December 2009 were eligible for this study. Eligibility criteria for this study included: 1) adenocarcinoma of the stomach; 2) T2 stage disease underwent gastrectomy with curative intent; 3) patients received at least modified D2 (D1 + 7, 8a, 9) or D2 lymph node dissection; 4) patients were completely followed-up; 5) no history of gastrectomy or other malignancy; 6) no history of neoadjuvant chemotherapy; and 7) no patients died during the initial hospital stay or for 1 month after surgery. As a result, 77 patients with modified D2 dissection and 118 patients with standard D2 dissection were enrolled in this study. To overall bias due to the different distribution of characteristics between patients accepted standard D2 dissection and those had modified D2 dissection, a case-control method using propensity score analysis was applied. We randomly selected 77 patients with standard D2 lymph node dissection as control group matched with the modified D2 group in baseline characteristics including gender, age at surgery, tumor location, tumor diameter, Borrmann type, histology, curability, TNM stage, types of gastrectomy, postoperative chemotherapy and complications.
Ultimately, 154 patients were included in the analysis, comprising 117 males (76.0%) and 37 females (24.0%). The age ranges from 21 to 85 years, and the median age was 60 years. All patients were categorized into 2 groups according to the extent of lymphadenectomy: the modified D2 group (mD2), patients accepted D1 lymphadenectomy with dissection lymph nodes along the left gastric, common hepatic and celiac arteries, including 77 patients; and the D2 group (D2), patients accepted standard D2 lymphadenectomy, including 77 patients.
Evaluation of clinicopathological variables and survival
Clinicopathological features studied included gender, age at surgery, tumor location, tumor size, Borrmann type, histology, curability, TNM stage, types of gastrectomy, extent of lymphadenectomy, postoperative complication and chemotherapy.
The tumors were staged according to the 7th edition of the UICC TNM Classification System, whereas lymphadenectomy and lymph node stations were defined according to the 3rd English Edition of the Japanese Classification of Gastric Carcinoma (14). Tumors were classified into two groups based on histology: differentiated type, including papillary, well or moderately differentiated adenocarcinoma, and undifferentiated type, including poorly differentiated or undifferentiated adenocarcinoma, signet ring cell carcinoma and mucinous carcinoma.
Follow-up
The patients were followed up every 3 months up to 2 years after surgery, then every 6 months up to 5 years, then every year or until death. Physical examination, laboratory tests, chest X-ray and abdominal ultrasound (US) were performed at each visit, while endoscopy and abdominal computed tomography (CT) were obtained every 6 months. The overall survival (OS) rate was calculated from the day of surgery until time of death or final follow-up. The median follow-up was 61 (range: 1–101) months for the matched pair. The date of final follow-up was December 30, 2015.
Statistical analysis
For continuous variables, parametric analysis was performed using Student’s t test. Categorical variables were analyzed by the Chi-square or Fisher’s exact test. OS curves were calculated using the Kaplan-Meier method based on the length of time between primary surgical treatment and final follow-up or death. The Log-rank test was used to assess statistical differences between curves. Independent prognostic factors were identified by the Cox proportional hazard regression model. P<0.05 (bilateral) was considered statistically significant. The statistical analysis was performed using the statistical analysis program package SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological characteristics
Of the 154 GC patients who underwent gastrectomy, 147 patients had an R0 resection, and 7 patients ended up with an R1 resection. Seventy-four patients received postoperative chemotherapy with 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX6). Postoperative complications during hospitalization included not only those directly associated with surgery, such as hemorrhage (1 case in mD2 group and 2 cases in D2 group), anastomotic leak (1 case in D2 group), pancreatic fistula (1 case in mD2 group and 3 cases in D2 group), and abdominal or wound infection (1 case in mD2 group and 2 cases in D2 group), but also those non-surgical ones, for example, pneumonia (6 cases in mD2 group and 4 cases in D2 group), deep venous thrombosis (1 case in mD2 groups) and urinary tract infection (1 cases in mD2 group and 3 cases in D2 group). Lymph node metastasis was observed in 43 patients, the metastatic rate was 27.9%. Metastatic status of each regional lymph node station is shown in Table 1. Station 3 was the most frequently invaded lymph node, followed by 4d, 4sb, 6, 7, 5, 8a, 9 and 1 station. Metastasis to 10, 11p, 11d and 12a lymph node station was rare in T2 stage GC.

Full table
All the patients were divided into two groups according to the extent of lymphadenectomy (Table 2). There were no statistical differences in gender, age at surgery, tumor location, tumor diameter, Borrmann type, histology, curability, TNM stage, types of gastrectomy, postoperative chemotherapy and complication between the two groups.

Full table
Survival analysis of all GC patients
The results of the univariate and multivariate analysis in all 154 patients are shown in Table 3. A total of 8 factors evaluated in the univariate analysis had a significant effect on survival: age at surgery, tumor location, tumor size, curability, TNM stage, type of gastrectomy, postoperative chemotherapy and complication. The extent of lymphadenectomy did not significantly affect OS in univariate survival analysis. The 5-year OS rate was 71.4% for patients in the mD2 group and 70.1% for those in the D2 group, respectively (P=0.690, Log-rank, Figure 1). In multivariate analysis, curability, tumor size, postoperative complication and TNM stage were found to be independent prognostic factors for OS (Table 3). Survival curves of independent prognostic factors are shown in Figure 2.

Full table
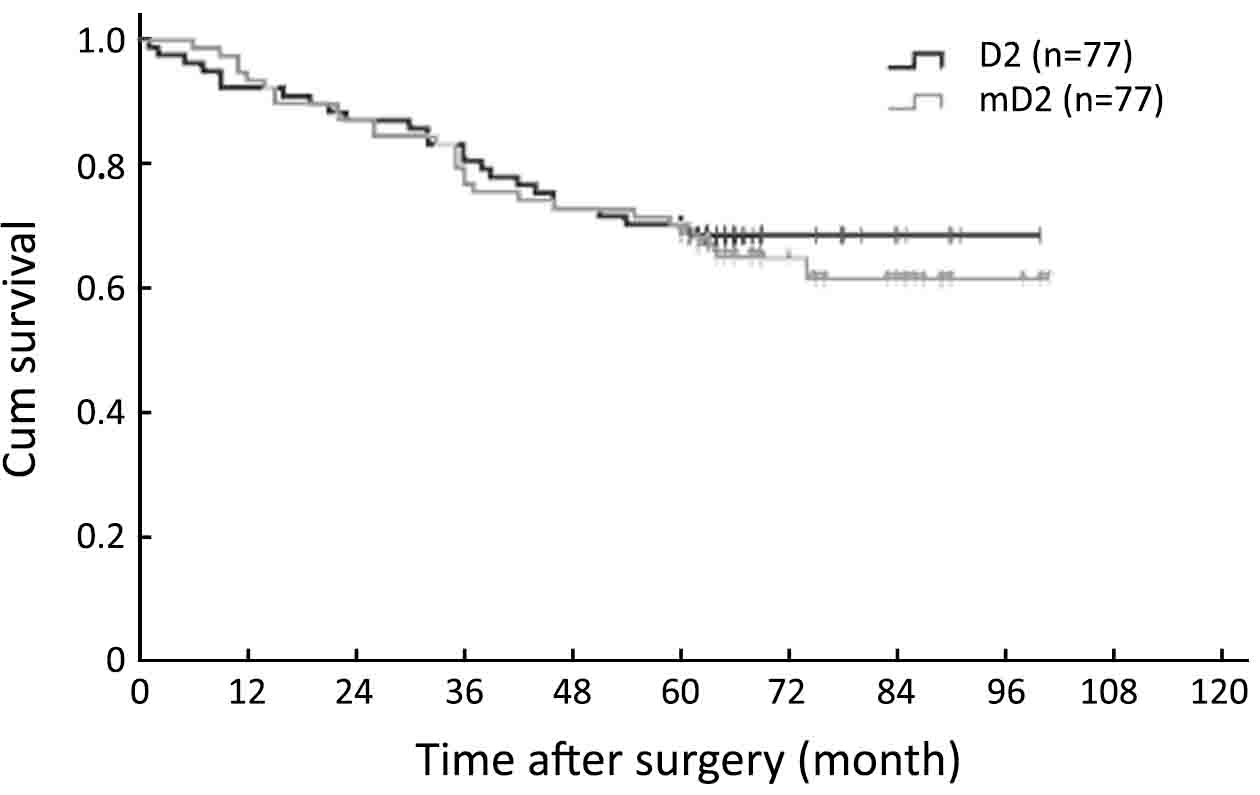

Surgical outcome of T2 stage GC
There were no statistical differences in postoperative complication, total number of metastatic lymph nodes and hospital stay after surgery between the two groups, whereas patients in the mD2 group tended to have less intraoperative blood loss (167.7±48.5 vs. 195.8±57.3 mL, P=0.001), lesser number of dissected lymph node (20.0±5.8 vs. 23.7±8.6, P=0.002) and shorter operation time (175.2±18.3 vs. 191.9±20.4 min, P<0.001) than those in the D2 group (Table 4).

Full table
Types of initial recurrence in T2 stage GC
The patterns and incidence of recurrence in the whole study series are shown in Table 5. Though patients in the mD2 group had a higher overall recurrence rate than those in the D2 group, the difference was not significant (40.3% vs. 36.4%, P=0.619). The recurrence types, including lymph node, gastric stump, anastomosis, gastric bed, peritoneal, hematogenous and combined recurrence, showed no significant difference between the two groups.

Full table
Discussion
GC is one of the most common malignancies worldwide and is a third leading cause of cancer-related death in China (19). Gastrectomy with D2 lymph node dissection is increasingly regarded as a standard treatment for locally advanced GC, while it might be an overtreatment for early GC with lower incidence of lymph node metastasis. Actually, according to the GC treatment guideline in Japan, gastrectomy with limited lymph node dissection is recommended as a curative treatment for cT1 early GC (1). It has been reported that T2 stage GC exhibited clinicopathological features similar to stage T1 GC and the prognosis of T2 stage GC after limited lymph node dissection was well (17). However, the rational extent of lymphadenectomy for T2 stage GC is still controversial.
In the present study, we found that the prognosis and recurrence patterns of T2 stage GC patients who had mD2 (D1 + 7, 8a and 9) lymph node dissection was similar to those undergoing standard D2 lymph node dissection, whereas patients with limited lymph node dissection tended to have less intraoperative blood loss, lesser number of lymph node retrieval and shorter operation time and hospital stay after surgery.
Japanese GC treatment guideline recommends that gastrectomy with D2 lymphadenectomy should be the standard surgical treatment for T2 stage GC. However, previous studies demonstrated that limited lymph node dissection could bring a good prognosis as extended D2 dissection in T2 GC (17,18). Ichikura et al. reported that D1 with dissection along the left gastric and common hepatic arteries (D1.5) resulted in a survival rate that was almost equal to that of D2 dissection in T2–3 stage GC, and thus suggested that use of D1.5 can be an attractive option in clinical trial (18). Tokunaga et al. even found the 5-year OS rate of T2 stage GC patients who were preoperatively diagnosed as early T1 GC and thus accepted limited lymph node dissection (D1 + 7, 8a, 9) was as high as 90.1%, which was similar to early GC. They concluded that gastrectomy with limited lymph node dissection could be indicated for T2 stage GC without obvious lymph node metastasis (17). In the present study, the 5-year OS rate was 71.4% for GC patients accepted mD2 and 70.1% for those accepted D2, respectively, and the difference was not statistically significant. Theoretically, an additional lymph node dissection is required for these with limited dissection to remove all second-tier lymph nodes. However, this additional treatment is not practical as the corresponding trauma and efficacy. Actually we discovered that 74 of 77 patients (96.1%) in the D2 group had no lymph node metastasis or lymph node metastasis within the pre-gastric area or in lymph nodes at station 7, 8a and 9, which could be dissected in mD2 dissection. This result was highly consistent with a previous study which indicated 98.1% of patients had lymph node metastasis with the pre-gastric area or in lymph nodes at station 7, 8a and 9 (17). We think the lower metastatic incidence in lymph nodes at station 10, 11p, 11d and 12a may account for the good prognosis of T2 stage GC patients undergoing limited lymph node dissection.
Usually, a limited D1 lymph node dissection increases locoregional recurrence, especially lymph node recurrence compared with extended D2 dissection as some metastatic lymph nodes are more or less left. The 15-year follow-up results of the randomized nationwide Dutch D1D2 trial suggested that locoregional recurrence rate of D1 dissection was remarkable as high as 41% comparing to 25% of D2 dissection (20). However, no significant difference in recurrence types and incidence was found between the two groups in this study, which may indicate that the lymph nodes at station 10, 11p, 11d and 12a were rarely invaded in fact.
Besides, we further demonstrated that patients with mD2 dissection tended to have less intraoperative blood loss and shorter operation time than those undergoing D2 dissection. Though our study did analyze the correlation between prognosis and the amount of blood loss or operation time, these factors have been reported to be associated with postoperative complication and had an adverse impact on survival for GC in previous studies (21-24).
Conclusions
Considering the increased intraoperative blood loss, prolonged operation time and no survival benefit of D2 dissection for T2 stage GC, the mD2 dissection may be a better option for T2 stage GC without obvious lymph node metastasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–19. [PubMed] DOI:10.1007/s10120-016-0622-4
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23–31. [PubMed] DOI:10.1002/bjs.9345
- Nitti D, Marchet A, Mocellin S, et al. Prognostic value of subclassification of T2 tumours in patients with gastric cancer. Br J Surg 2009;96:398–404. [PubMed] DOI:10.1002/bjs.6487
- Liu X, Long Z, Cai H, et al. Analysis of lymph node metastasis correlation with prognosis in patients with T2 gastric cancer. PLoS One 2014;9:e105112. [PubMed] DOI:10.1371/journal.pone.0105112
- Kim YI, Lee JH, Kook MC, et al. Lymph node metastasis risk according to the depth of invasion in early gastric cancers confined to the mucosal layer. Gastric Cancer 2016;19:860–8. [PubMed] DOI:10.1007/s10120-015-0535-7
- Lee JH, Choi IJ, Han HS, et al. Risk of lymph node metastasis in differentiated type mucosal early gastric cancer mixed with minor undifferentiated type histology. Ann Surg Oncol 2015;22:1813–9. DOI:10.1245/s10434-014-4167-7
- de Steur WO, Dikken JL, Hartgrink HH. Lymph node dissection in resectable advanced gastric cancer. Dig Surg 2013;30:96–103. [PubMed] DOI:10.1159/000350873
- Eom BW, Joo J, Kim YW, et al. Is there any role of additional retropancreatic lymph node dissection on D2 gastrectomy for advanced gastric cancer?. Ann Surg Oncol 2013;20:2669–75. [PubMed] DOI:10.1245/s10434-013-2938-1
- Tamura S, Takeno A, Miki H. Lymph node dissection in curative gastrectomy for advanced gastric cancer. Int J Surg Oncol 2011;2011:748745. [PubMed] DOI:10.1155/2011/748745
- Eom BW, Joo J, Kim YW, et al. Improved survival after adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for advanced distal gastric cancer. Surgery 2014;155:408–16. [PubMed] DOI:10.1016/j.surg.2013.08.019
- Tokunaga M, Ohyama S, Hiki N, et al. Therapeutic value of lymph node dissection in advanced gastric cancer with macroscopic duodenum invasion: is the posterior pancreatic head lymph node dissection beneficial?. Ann Surg Oncol 2009;16:1241–6. [PubMed] DOI:10.1245/s10434-009-0345-4
- Sasako M, McCulloch P, Kinoshita T, et al. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 1995;82:346–51. [PubMed]
- Liang Y, Wu L, Wang X, et al. Positive impact of adding No.14v lymph node to D2 dissection on survival for distal gastric cancer patients after surgery with curative intent. Chin J Cancer Res 2015;27:580–7. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.12.02
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12. [PubMed] DOI:10.1007/s10120-011-0041-5
- Bu Z, Zheng Z, Li Z, et al. Lymphatic vascular invasion is an independent correlated factor for lymph node metastasis and the prognosis of resectable T2 gastric cancer patients. Tumour Biol 2013;34:1005–12. [PubMed] DOI:10.1007/s13277-012-0637-3
- Komatsu S, Ichikawa D, Kurioka H, et al. Prognostic and clinical evaluation of patients with T2 gastric cancer. Hepatogastroenterology 2005;52:965–8. [PubMed]
- Tokunaga M, Hiki N, Fukunaga T, et al. Better prognosis of T2 gastric cancer with preoperative diagnosis of early gastric cancer. Ann Surg Oncol 2009;16:1514–9. [PubMed] DOI:10.1245/s10434-009-0404-x
- Ichikura T, Chochi K, Sugasawa H, et al. Modified radical lymphadenectomy (D1.5) for T2-3 gastric cancer. Langenbecks Arch Surg 2005;390:397–402. [PubMed] DOI:10.1007/s00423-005-0570-7
- Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1–11. [PubMed] DOI:10.3978/j.issn.1000-9604.2016.02.08
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49. [PubMed] DOI:10.1016/S1470-2045(10)70070-X
- Jiang N, Deng JY, Ding XW, et al. Effect of complication grade on survival following curative gastrectomy for carcinoma. World J Gastroenterol 2014;20:8244–52. [PubMed] DOI:10.3748/wjg.v20.i25.8244
- Liang YX, Guo HH, Deng JY, et al. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol 2013;19:5542–50. [PubMed] DOI:10.3748/wjg.v19.i33.5542
- Dhar DK, Kubota H, Tachibana M, et al. Long-term survival of transmural advanced gastric carcinoma following curative resection: multivariate analysis of prognostic factors. World J Surg 2000;24:588-93; discussion 593-4.
- Kamei T, Kitayama J, Yamashita H, et al. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg 2009;33:1240–6. [PubMed] DOI:10.1007/s00268-009-9979-4
