Adjuvant chemotherapy may improve outcome of patients with non-small-cell lung cancer with metastasis of intrapulmonary lymph nodes after systematic dissection of N1 nodes
Introduction
Accurate pathologic examination is essential to the staging of non-small-cell lung cancer (NSCLC) because it can indicate oncologic outcomes and instruct postoperative adjuvant treatment (1). Recently, retrieval of intrapulmonary lymph nodes (IPLNs) has garnered increasing research interest (2-4). A mean of five intralobar nodes discovered from a lobe specimen can reveal important clinical information with regard to staging and adjuvant therapy (5,6).
The clinical importance of IPLNs has been demonstrated from two aspects. First, harvesting more metastatic nodes might enable very accurate pathologic staging due to IPLN retrieval (though this procedure is usually neglected in current clinical practice) (7,8). Second, systematic retrieval and thorough examination of IPLNs may confirm upstaging of only 11% of patients to pN1 and most patients remain as pN0, which raises questions about the survival benefit of this procedure and the treatment protocol followed (9). Recently, we reported that omission of IPLN retrieval might have an inferior impact on outcome evaluation in patients with pN0 status (10).
Detailed analyses about the distribution pattern of involved nodes and related clinical characteristics (especially through systematic dissection of five-level N1 nodes) are lacking. Also, patients with node involvement limited to level 13−14 have an intermediate outcome between those with pN0 and those with pN1 status (involvement of level 10 to level 12) (11). Adjuvant treatment plans and the related outcomes for this specific N1 cohort merit further investigation.
We investigated the metastatic features of IPLNs and explored the survival benefit of adjuvant chemotherapy in patients whose metastatic nodes were limited to intrapulmonary stations.
Materials and methods
Patient eligibility
The Institutional Review Board of Peking University Cancer Hospital (Beijing, China) approved the study protocol and waived the need for written informed consent by each patient because of the retrospective nature of the study.
From January 2006 to December 2014, 1,979 patients admitted to Peking University Cancer Hospital were diagnosed with lung cancer. They underwent lung resection and systematic dissection of lymph nodes and IPLN retrieval. All cases diagnosed as “pathologic N1” were collected retrospectively and analyzed. Patients with non-primary lung cancer or carcinoid tumor were not included in our study. The clinical and pathologic information of this cohort was collected and analyzed prospectively.
Diagnostic procedure, surgery, and pathologic examination
Preoperative staging procedures were undertaken according to guidelines set by the National Comprehensive Cancer Network (NCCN). Routine preoperative staging included computed tomography (CT) of the chest, magnetic resonance imaging (MRI) of the brain, abdominal ultrasonography, bone scintigraphy, or positron emission tomography (PET)/CT. The type of pulmonary resection (i.e., lobectomy, bilobectomy, sleeve-lobectomy or pneumonectomy) was decided according to the tumor location.
Patients underwent systematic mediastinal lymphadenectomy according to the requirements of NCCN guidelines: dissection of at least three stations of N2 nodes, including station 7 (subcarinal lymph node) (12). Definition of lymph node stations was based on the nomenclature of the International Association for the Study of Lung Cancer (IASLC) (1). Pathologic staging is described in the TNM Classification of Malignant Tumors, 7th Edition (13).
For the N1 stations, surgeons collected lymph nodes from stations 10−12 during resection and labeled them separately. The remaining part of station 12 (if there was any left), the segmental station 13 and subsegmental station 14 was retrieved routinely when the specimen was removed from the patient, and was labeled according to the location of the attached segments and subsegments (14).
Tissues and lymph nodes were sent for routine pathology with paraffin blocks. Lymph nodes were bivalved along their longitudinal axis and submitted in total for microscopic evaluation. Small nodes (≤0.4 cm) were submitted without bivalving. A single hematoxylin and eosin-stained slide was prepared from each block.
For patients with positive nodes limited in the intrapulmonary area (level 12−14), a four-cycle platinum-based regimen is suggested. However, due to a lack of evidence in this group, some patients might not comply and receive only routine follow-up. The treatment plan was recorded and compared between those receiving chemotherapy and those not receiving chemotherapy.
All patients were evaluated postoperatively with chest CT scan, and cervical/abonominal B-ultrasound and brain MRI, when clinically indicated, in addition to periodic clinical follow-up, in accordance with NCCN guidelines. The main outcome indicator of the study is 5-year overall survival (OS) and disease-free survival (DFS).
Statistical analysis
Depending on the normality of distribution, the Student’s t-test or Wilcoxon rank test was used to compare continuous variables between matched groups. Median value was used for describing lymph node number for the non-normal distribution, and x±s was used for number of normal distribution. The Pearson’s Chi-square test or Fisher’s exact test was used to compare proportions, as required. Survival was estimated using the Kaplan-Meier method, and the significance of differences determined using the log-rank test. All statistical tests were two-sided, with a significance level set at 0.05. IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA) was used for statistical analyses.
Results
Patient characteristics
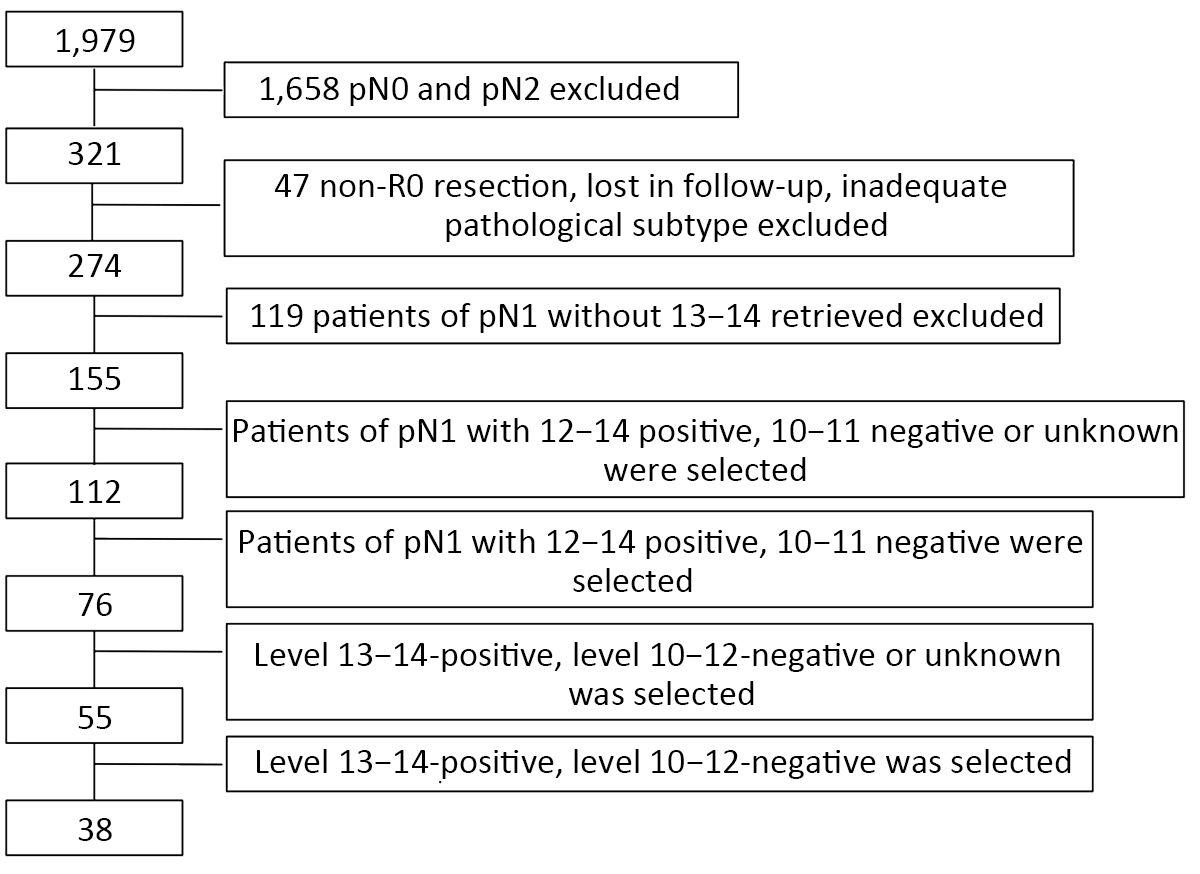
During the investigation period, 1,979 patients underwent surgery, 1,658 patients were excluded for the pathological N2 status, 47 patients were excluded for non-R0 resection, lost in follow-up or inadequate pathological subtype, 119 patients were excluded for absence of 13−14 retrieved, and then 155 patients were involved in the final analysis. The final follow-up was conducted in December 2017, the patients including and excluding criteria was shown in the flow chart (Figure 1).
The median duration of follow-up of the entire cohort was 47.3 (range, 8−130) months. No patient died within the perioperative period and no patient was lost to follow-up. The median age at surgery in this cohort was 58 (range, 29−78) years, and the male:female ratio was 1.92:1. The entire cohort comprised 80 cases of right-lung cancer and 75 cases of left-lung cancer. Pathology revealed 96 cases of adenocarcinoma, 50 of squamous cell carcinoma, 4 of adenosquamous carcinoma, 2 of large cell carcinoma, and 3 of mixed type.
Retrieval and involvement of IPLNs
We summarized the baseline information about lymphadenectomy in our cohort. The total median number of N2 stations examined and median number of N2 nodes harvested were 4 stations and 12 lymph nodes; for N1 nodes, they were 3 stations and 10 lymph nodes, respectively. Among all patients: 140 cases (90.3%) had level-12 lymph nodes collected, with a median number of 3 nodes harvested; 152 cases (98.1%) had level-13 lymph nodes collected, with a median number of 3 nodes harvested; 64 (41.3%) had level-14 lymph nodes collected, with a median number of 2 nodes harvested.
Involvement of a single station at levels 10, 11, 12, 13, and 14 was observed in 13 (8.4%), 7 (4.5%), 28 (18.1%), 42 (27.1%) and 8 (5.2%) cases, respectively. Involvement of multiple stations was noted in 57 (36.8%) cases.
Among the entire group, IPLN involvement (level 12−14-positive, level 10−11-negative or unknown) was found in 112 (72.3%) cases and the other 43 (27.7%) cases showed extrapulmonary N1 involvment (level 10−11-positive, level 12−14-postive or negative). The median level and number of IPLN involvement in patients with IPLN involvement was 1 and 1, respectively. Compared with the extrapulmonary N1 group (level 10−11-positive), the clinical features of the intrapulmonary N1 group (level 12−14-positive) were more likely to be of a squamous subtype (P=0.024), with less lymphovascular invasion (P=0.003) and less pleural invasion (P=0.002).
Impact of adjuvant chemotherapy on IPLN metastasis
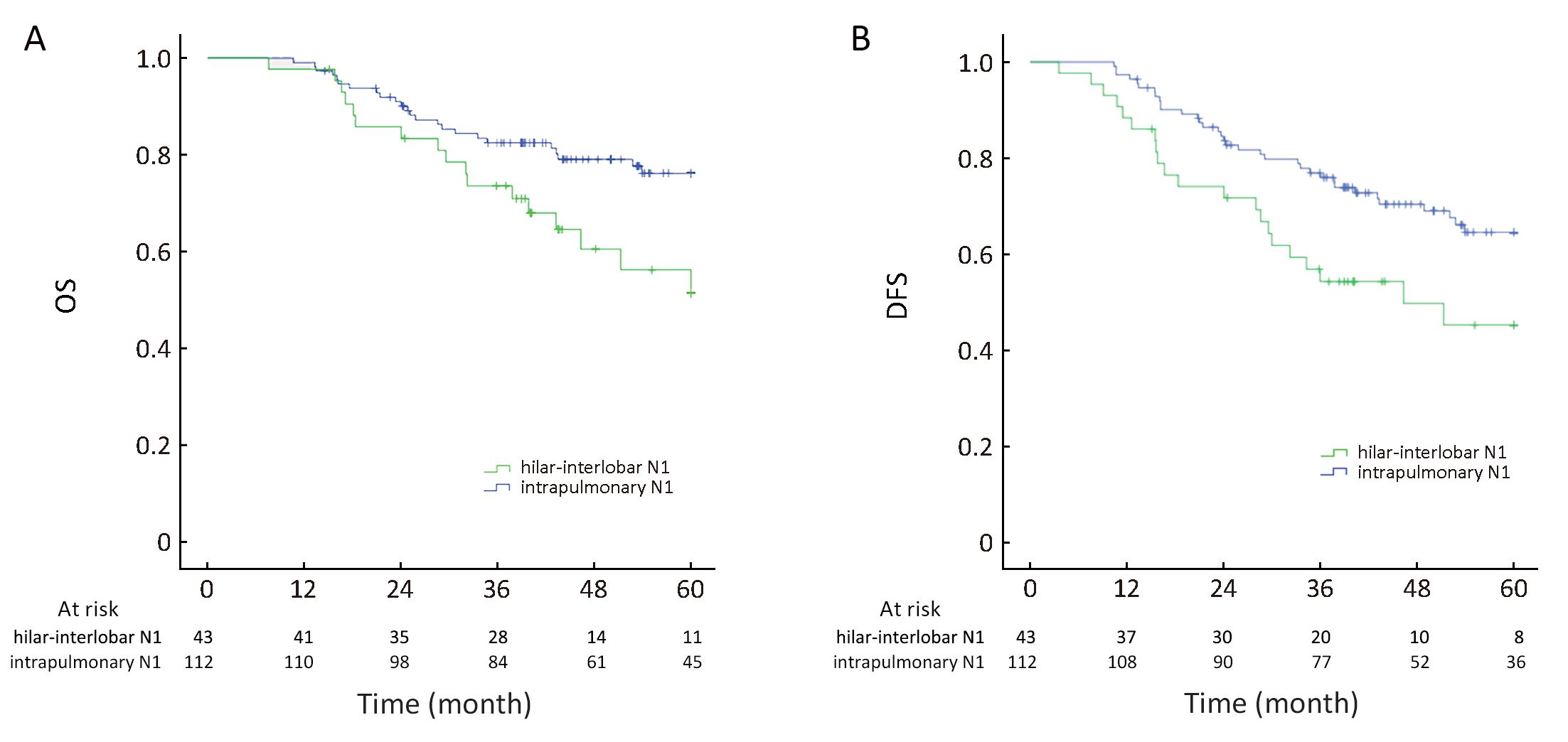
Based on the highest level of lymph-node involvement, the entire cohort was divided into the “extrapulmonary N1” group (involvement of level 10−11 in 43 cases) and “intrapulmonary N1” group (disease spread between level 12 and level 14 in 112 cases). Patients in the extrapulmonary N1 group showed inferior outcome compared with patients in the intrapulmonary N1 group (5-year OS: 47.3±2.7 vs. 52.7±1.4 months, P=0.017; 5-year DFS: 40.8±3.2 vs. 49.1±1.6 months, P=0.015) (Figure 2).
For the intrapulmonary N1 group (level 12−14-positive, level 10−11-negative or unknown, n=112), 62 patients received four cycles of platinum-based adjuvant chemotherapy and 50 refused adjuvant therapy but received routine follow-ups. Clinical and pathologic parameters were well balanced between the two groups in terms of sex, age, T staging, tumor diameter, pleural invasion and lymphovascular invasion. A significant difference was not found in OS or DFS at 5 years between the chemotherapy group and non-chemotherapy group (5-year OS: 54.6±1.6 vs. 50.4±2.4 months, P=0.177) (Figure 3A).
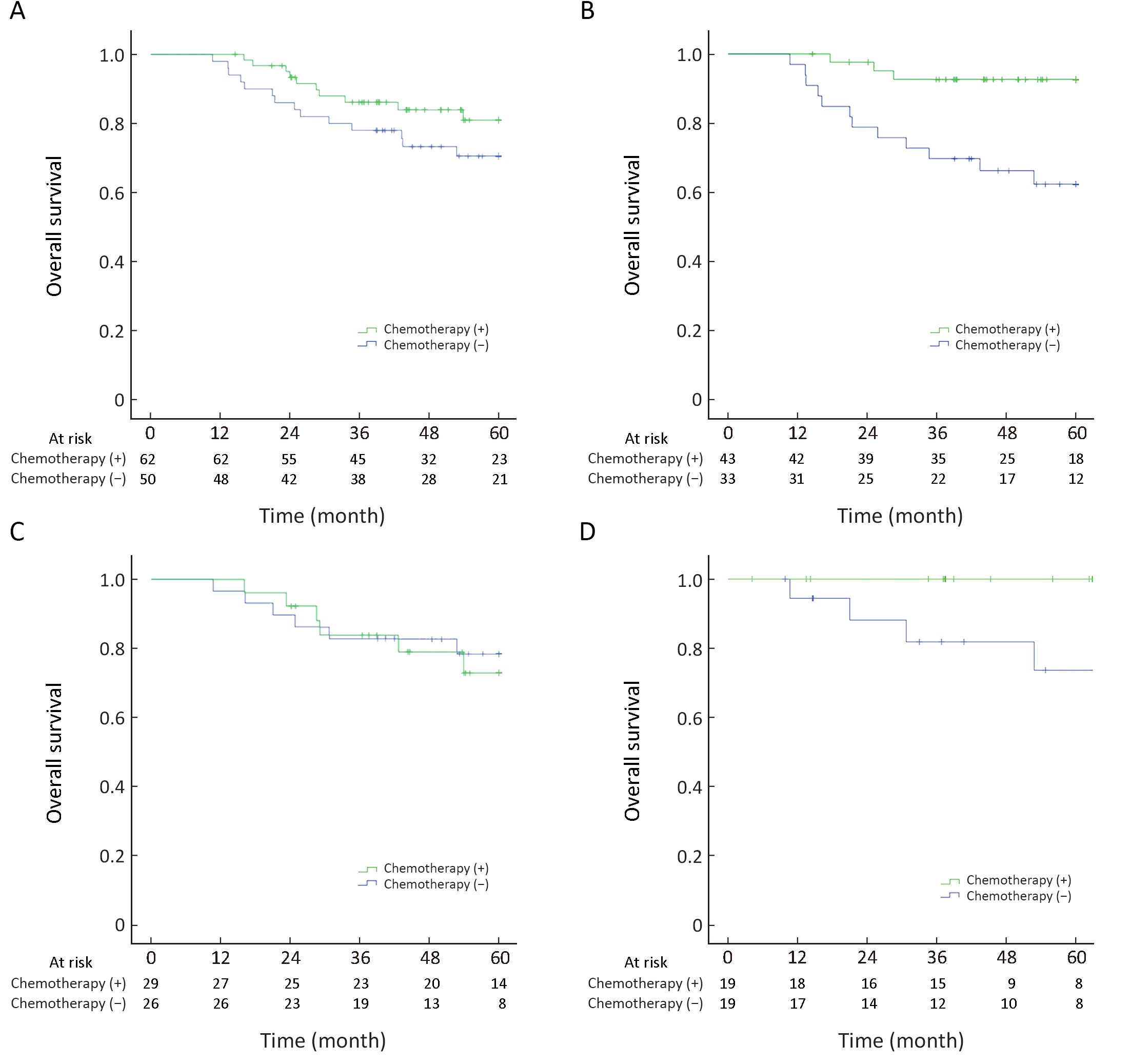
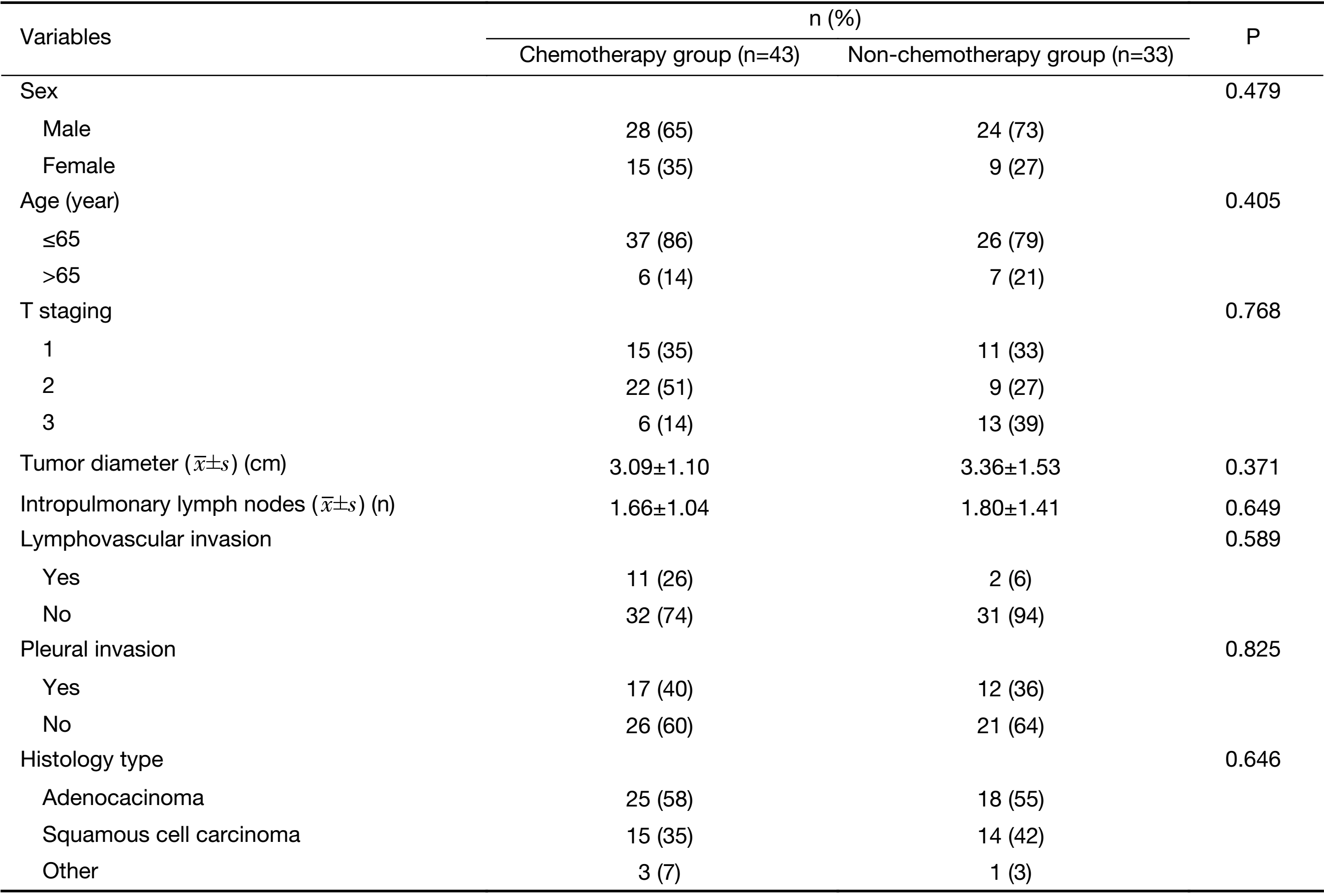
Furthermore, among these 112 cases, the pathology reports of 76 cases showed that level-10 and level-11 nodes had been harvested without cancer involvement (level 12−14-positive, level 10−11 both negative). Forty-three cases had adjuvant chemotherapy and the other 33 patients did not receive adjuvant therapy (Table 1). The 5-year OS for patients receiving adjuvant chemotherapy was better than that for cases not receiving adjuvant chemotherapy (5-year OS: 57.3±1.5 vs. 47.1±3.2 months, P=0.002) (Figure 3B). All variates (age, sex, tumor location, T staging, histology, lymphovascular invasion, visceral pleural invasion and adjuvant chemotherapy) were examined in multivariate Cox models among this group. Adjuvant chemotherapy was confirmed to be an independent and significant prognostic indicator for 5-year OS, with a hazard ratio of 0.266 (0.095−0.745, P=0.012).
Full table
Among the intrapulmonary N1 group, 55 patients with metastatic nodes were found to have them only at level 13−14, with level 10−12 being negative or not recorded at one or more levels (level 13−14-positive, level 10−12-negative or unknown). The impact of adjuvant chemotherapy on these patients was investigated further. Twenty-six patients received four cycles of adjuvant chemotherapy and 29 refused. The log-rank test revealed no significance in 5-year OS between the chemotherapy group and non-chemotherapy group (5-year OS: 53.1±1.9 vs. 52.9±2.8 months, P=0.790) (Figure 3C).
Similarly, 38 of these 55 cases had lymph-node metastasis limited only to level 13−14, and pathology reports showed complete examination from level 10 to level 12 with none of these three levels involved (level 13−14-positive, level 10−12 all negative). Nineteen cases had adjuvant chemotherapy and the other 19 patients did not receive adjuvant therapy. The 5-year OS for patients who had adjuvant chemotherapy was better than those who had not (58.3±1.7 vs. 51.0±4.2 months, P=0.048) (Figure 3D). Cox regression was performed and all variates (age, sex, tumor location, T staging, histology, visceral pleural invasion, differentiation and adjuvant chemotherapy) were examined in multivariate Cox models, and adjuvant chemotherapy was suggested as a favorable factor for 5-year OS (P=0.037).
Discussion
According to the IASLC nodal chart, systematic investigation of regional lymph nodes is suggested for accurate staging (1). However, current practice guidelines recommend sampling or dissection at a minimum of three stations of mediastinal lymph nodes plus three stations of N1 nodes during surgery (13,15). The discrepancy between the “ideal” recommendation and real-world practice has shown the clinical importance of IPLNs, and studies have disclosed the role of IPLNs in staging and outcome assessment (10). Here, we demonstrated the distribution of IPLNs in detail and validated the prognostic significance of chemotherapy in this specific subgroup.
Our study suggested that 72.3% of the N1 cohort had involvement of level 12−14. Data for disease involvement in IPLNs reveal the necessity of IPLN retrieval. Furthermore, detailed data from thorough examination of lymph nodes may facilitate revision of N staging based on the latest version of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging manual.
The oncologic outcome of pN1 patients can be evaluated by several methods (16,17). Data from the IASLC Lung Cancer Staging Project have suggested that it might be appropriate to subdivide the current N staging descriptors into N1a (single N1 zone) and N1b (multiple N1 zones) (1). However, considering this anatomic heterogeneity, several studies have sought to investigate further the anatomic stations within the N1 classification in the hope of refining the nodal staging schema for better prognostic stratification. For example, Rena and colleagues reported the highest involvement of N1 stations to be an independent prognostic factor (11). Several studies have identified a trend towards worse outcomes with the involvement of more anatomically central nodes (11). Both types of staging method can aid better understanding of the diverse outcomes of N1 patients.
Hilar and interlobar lymph nodes can be havested readily intraoperatively, including during video-assisted thoracoscopic surgery (18). Level-10 hilar nodes are in close proximity to N2 mediastinal nodal basins (19,20), and involvement of these nodes can facilitate disease spread to the mediastinum. Level-12−14 nodes are encased peripherally within the parenchyma and can be removed in their entirety only during anatomic lung resection (21). The discouraging fact is that deeply located nodes from level 13 and level 14 are easily negelected in routine practice.
Our data also demonstrated that survival for patients with pN1 (level 10−11) was inferior to that for cases with pN1 (level 12−14), results that are in accordance with those of Rena and colleagues (22). On the contrary, we did not observe a survival difference between the involvement of single and multiple N1 stations (data not shown). The reason might be that more detailed data of disease spread to level 13−14 were evaluated in this cohort, and IPLN involvement might present superior outcomes irrespective of whether single or multiple stations are involved. Additional clinical trials are required to express the N descriptor more accurately.
Patients with lung cancer diagnosed with pN1 were recomended for adjuvant chemotherapy according to NCCN guidelines (23). However, most published data are based on studies with metastasis to level-10 and level-11 lymph nodes (1). Furthermore, patients with IPLN involvement represented 72.3% of the N1 cohort in our study. Therefore, more evidence is needed to demonstrate the survival benefit of adjuvant chemotherapy for patients with IPLN involvement (24). First, we chose an unselected cohort with IPLN metastasis for comparison. The criteria for inclusion is that pathology reports showed positivity in intrapulmonary levels but that level 10−11 might be negative or not recorded. Interestingly, survival benefit was not significantly different between the adjuvant chemotherapy group and those not receiving chemotherapy in this unselected cohort. However, if we limited the selection criteria further to patients whose pathology reports confirmed negativity at level 10 and level 11, we found that adjuvant chemotherapy could yield significantly better survival outcome compared with those not receiving chemotherapy in this selected cohort. Therefore, incomplete or suboptimal examination at N1 nodes could result in potential bias for outcome evaluation of adjuvant chemotherapy for patients with intrapulmonary N1 metastasis. This result emphasized the necessity of systematic dissection of N1 nodes at multiple levels.
We also tested if a similar trend occurred in patients whose metastatic nodes were limited only to level 13 and/or level 14. Again, among the unselected cohort, survial benefit was not significantly different between the two groups. However, upon complete examination of nodes from level 10 to level 12, adjuvant chemotherapy showed survival benefit compared with those not having chemotherapy. This result reiterates the importance of systematic dissection of N1 nodes according to the IASLC nodal chart, and may supplement an indication of adjuvant chemotherapy for this special N1 subgroup.
A retrospective analysis, small sample size and non-randomization were the major limitations of our study that could have resulted in intergroup bias. For patients with IPLN involvement, although the clinical-pathologic parameters were balanced in the two subgroups, empirical experience and lack of high-level evidence in this special cohort might result in more subjective decision-making with regard to adjuvant chemotherapy. Also, in the present study, the extent and nomenclature of IPLNs during retrieval were based on the experience of a singe center (though a standardized training process and definition of lymph node during retrieval has been carried out since the beginning of data collection). Multicenter-based clinical trials are needed to validate the clinical relevance of IPLN retrieval and the prognostic value of adjucant chemotherapy for this N1 group.
Conclusions
A high prevalence of IPLN metastasis may indicate the clinical relevance of IPLN retrieval. The oncologic outcome may be improved by adjuvant chemotherapy for patients receiving systematic dissection of N1 nodes and whose metastatic nodes are limited to only intrapulmonary stations.
Acknowledgements
This work was supported in part by National Key R&D Program of China (No. 2018YFC0910700); the Beijing Municipal Administration of Hospitals’ Youth Programme (No. QML20171103); the Special Fund of Beijing Municipal Administration of Hospitals Clinical Medicine Development (No. XMLX201841); the Beijing Municipal Science & Technology Commission (No.Z161100000516063); and Beijing Human Resources and Social Security Bureau (Beijing Millions of Talents Project, 2018A05).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhang L, Ou W, Liu Q, et al. Pemetrexed plus carboplatin as adjuvant chemotherapy in patients with curative resected non-squamous non-small cell lung cancer. Thorac Cancer 2014;5:50–6. [PubMed] DOI:10.1111/1759-7714.12058
- Riquet M, Mordant P. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. Transl Lung Cancer Res 2013;2:1–2. [PubMed] DOI:10.3978/j.issn.2218-6751.2012.09.02
- Maeshima AM, Tsuta K, Asamura H, et al. Prognostic implication of metastasis limited to segmental (level 13) and/or subsegmental (level 14) lymph nodes in patients with surgically resected nonsmall cell lung carcinoma and pathologic N1 lymph node status. Cancer 2012;118:4512–8. [PubMed] DOI:10.1002/cncr.27424
- Osarogiagbon RU, Decker PA, Ballman K, et al. Survival implications of variation in the thoroughness of pathologic lymph node examination in American College of Surgeons Oncology Group Z0030 (Alliance). Ann Thorac Surg 2016;102:363–9. [PubMed] DOI:10.1016/j.athoracsur.2016.03.095
- Osarogiagbon RU, Hilsenbeck HL, Sales EW, et al. Improving the pathologic evaluation of lung cancer resection specimens. Transl Lung Cancer Res 2015;4:432–7. [PubMed] DOI:10.3978/j.issn.2218-6751.2015.07.07
- Liang W, He J, Shen Y, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: A population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol 2017;35:1162–70. [PubMed] DOI:10.1200/JCO.2016.67.5140
- Li ZX, Yang H, She KL, et al. The role of segmental nodes in the pathological staging of non-small cell lung cancer. J Cardiothoracic Surg 2013;8:225. [PubMed] DOI:10.1186/1749-8090-8-225
- Fukui T, Kato K, Okasaka T, et al. Predictors for hilar/intrapulmonary lymph node metastasis in discrete type of clinical N1 non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2017;65:640–5. [PubMed] DOI:10.1007/s11748-017-0827-4
- Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30:2823–8. [PubMed] DOI:10.1200/JCO.2011.39.2589
- Wang X, Yan S, Lv C, et al. Impact of omission of intrapulmonary lymph node retrieval on outcome evaluation of lung cancer patients without lymph node metastasis: A propensity score matching analysis. Clin Lung Cancer 2017;18:e411–e416. [PubMed] DOI:10.1016/j.cllc.2017.05.001
- Rena O, Boldorini R, Papalia E, et al. Metastasis to subsegmental and segmental lymph nodes in patients resected for non-small cell lung cancer: prognostic impact. Ann Thorac Surg 2014;97:987–92. [PubMed] DOI:10.1016/j.athoracsur.2013.11.051
- Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25–33. [PubMed] DOI:10.1016/j.lungcan.2005.01.001
- Ettinger D, Johnson B. Update: NCCN small cell and non-small cell lung cancer Clinical Practice Guidelines. J Natl Compr Canc Netw 2005;3(Suppl 1):S17–21. [PubMed]
- Li ZX, Yang H, She KL, et al. The role of segmental nodes in the pathological staging of non-small cell lung cancer. J Cardiothorac Surg 2013;8:225. [PubMed] DOI:10.1186/1749-8090-8-225
- Wang X, Yan S, Phan K, et al. Mediastinal lymphadenectomy fulfilling NCCN criteria may improve the outcome of clinical N0-1 and pathological N2 non-small cell lung cancer. J Thorac Dis 2016;8:342–9. [PubMed] DOI:10.21037/jtd.2016.02.49
- Mordant P, Pricopi C, Legras A, et al. Prognostic factors after surgical resection of N1 non-small cell lung cancer. Eur J Surg Oncol 2015;41:696–701. [PubMed] DOI:10.1016/j.ejso.2014.10.003
- Citak N, Sayar A, Metin M, et al. The prognostic significance of metastasis to lymph nodes in aortopulmonary zone (Stations 5 and 6) in completely resected left upper lobe tumors. Thorac Cardiovasc Surg 2015;63:568–76. [PubMed] DOI:10.1055/s-0035-1546463
- Fan JQ, Yao J, Chang ZB, et al. Left upper lobectomy and systematic lymph nodes dissection in enlarged pulmonary hilar lymph nodes in primary lung cancer patient by uniportal video-assisted thoracic surgery. J Thorac Dis 2016;8:2259–63. [PubMed] DOI:10.21037/jtd.2016.01.80
- Okada M, Sakamoto T, Yuki T, et al. Border between N1 and N2 stations in lung carcinoma: lessons from lymph node metastatic patterns of lower lobe tumors. J Thorac Cardiovasc Surg 2005;129:825–30. [PubMed] DOI:10.1016/j.jtcvs.2004.06.016
- Asamura H, Suzuki K, Kondo H, et al. Where is the boundary between N1 and N2 stations in lung cancer?. Ann Thorac Surg 2000;70:1839. [PubMed]
- Haney JC, Hanna JM, Berry MF, et al. Differential prognostic significance of extralobar and intralobar nodal metastases in patients with surgically resected stage II non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:1164–8. [PubMed] DOI:10.1016/j.jtcvs.2013.12.015
- Rena O, Boldorini R, Papalia E, et al. Metastasis to subsegmental and segmental lymph nodes in patients resected for non-small cell lung cancer: prognostic impact. Ann Thorac Surg 2014;97:987–92. [PubMed] DOI:10.1016/j.athoracsur.2013.11.051
- Liu Y, Zhai X, Li J, et al. Is there an optimal time to initiate adjuvant chemotherapy to predict benefit of survival in non-small cell lung cancer? Chin J Cancer Res 2017;29:263-71. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.03.12
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351–60. [PubMed] DOI:10.1056/NEJMoa031644