Exploratory clinical study of chidamide, an oral subtype-selective histone deacetylase inhibitor, in combination with exemestane in hormone receptor-positive advanced breast cancer
Introduction
It is shown that 50%−70% of breast cancers are estrogen receptor (ER) positive and/or progesterone receptor (PR) positive (1). Endocrine therapies are the foundation for treatment of these hormone receptor-positive (HR+) cancers. Despite advances in endocrine therapy, either primary or secondary drug resistance is a common issue in patients with the treatment, leading to disease progression or recurrence (2). Although the intrinsic mechanisms of endocrine resistance are incompletely uncovered, ER dysfunction (silence, hypersensitivity, mutation, etc.), alternative activation of growth signaling pathways, and/or altered epigenetic modifications are suggested by preclinical and clinical studies (3-5).
Histone deacetylase (HDAC) inhibitors are a promising drug class in overcoming resistance to endocrine therapy. In general, those compounds induce cell proliferation arrest, differentiation, and cell death in breast cancer cells (6). Also, HDAC inhibitors, such as vorinostat and entinostat, have been shown to restore hormone sensitivity via down-regulation of estrogen-independent growth factor signaling pathways, normalization of ER levels, and increase in aromatase enzyme levels in vitro and preclinical studies (7-9). Furthermore, in a randomized phase II trial the addition of entinostat to exemestane showed a potential prolonged progression-free survival (PFS) and overall survival (OS) in patients with previously treated hormone-sensitive metastatic breast cancer (10).
Chidamide (CS055/Tucidinostat/Epidaza®) is an oral benzamide class of HDAC inhibitor with broad antitumor activities by selectively inhibiting HDAC1, 2, 3 and 10 (11), and has been approved by the China Food and Drug Administration (CFDA) as a treatment for relapsed or refractory peripheral T cell lymphoma (PTCL) (12). Previous studies have shown that benzamide class of HDAC subtype-selective inhibitors, including chidamide and entinostat, enhance tumor immune surveillance via activation of natural killer (NK) cell and antigen-specific cytotoxic T lymphocyte (CTL)-mediated cellular anti-tumor immunity, which differentiates them from other non-selective HDAC inhibitors (13-16). Chidamide has also been demonstrated to down-regulate estrogen-independent growth factor signaling pathways and restore the sensitivity to anti-estrogen agents in preclinical investigations (17).
Exemestane is a steroidal aromatase inhibitor (AI) with well-established efficacy in HR+, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer (ABC) patients progressed on previous endocrine therapy, including tamoxifen and/or nonsteroidal AIs (i.e., letrozole or anastrozole). This exploratory clinical study was thus performed to evaluate the safety, pharmacokinetics (PK), and preliminary efficacy of chidamide in combination with exemestane in postmenopausal women with HR+ and HER2-negative ABC that recurrent or progressed to at least one endocrine therapy.
Materials and methods
Study design
The study design was a single-arm, open-label, exploratory trial of chidamide in combination with exemestane in HR+ ABC patients. The Institutional Review Board at each participating center approved the study, which was conducted by the principles of Good Clinical Practice, the provisions of the Declaration of Helsinki, and other applicable local regulations. The study was registered on ClinicalTrials.gov (No. NCT02482753).
The primary objective was to determine the safety and PK profiles of chidamide with exemestane in ABC patients. The secondary goal was to evaluate the preliminary efficacy, including objective response rate (ORR) and PFS.
In the 4-day-run-in period, patients received single-agent exemestane 25 mg (provided by Pfizer Inc.) on d 1 and chidamide 30 mg (supplied by Shenzhen Chipscreen Biosciences, Ltd.) on d 2. From d 5, patients started combination treatment with exemestane 25 mg daily and chidamide 30 mg twice a week (BIW). A treatment cycle was defined as 28 d.
Before study entry, patients underwent a complete history and physical examination, and all prior anticancer treatments and their residual side effects were recorded, if any. Baseline imaging evaluations were obtained to define the extent of disease. Baseline laboratory tests included a complete blood cell, platelet counts and blood chemistry. Physical examination, laboratory assessments and radiological evaluations were repeated every 8 weeks. Toxicities were graded using the National Cancer Institute Common Toxicity Criteria (NCICTC), version 4.0.
Patients continued on treatment until they had disease progression, or were unable to tolerate with therapy, or withdrew consent.
Patients and eligibility criteria
Written informed consent was obtained from all patients before enrollment. Eligible patients included: 1) 18 to 75 years old postmenopausal women and had an Eastern Cooperative Oncology Group performance status of 0 or 1; 2) ER+, HER2-negative advanced or metastasis breast cancer patients who were experiencing disease relapse or progression after at least one endocrine therapy; 3) only one prior line of chemotherapy in the metastatic setting was permitted; 4) the total number of endocrine therapy and chemotherapy did not exceed 4 regimens; and 5) disease had to be measurable by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 or non-measurable bone-only disease.
Exclusion criteria mainly included 1) prior treatment with exemestane; 2) presence of visceral crisis; 3) evidence or history of central nervous system (CNS) metastasis; 4) cancer chemotherapy, radiation therapy, or any investigational agent within 4 weeks before the baseline evaluations; 5) an active severe infection; 6) unstable angina within 6 months; 7) myocardial infarction within 12 months; 8) therapy for life-threatening ventricular arrhythmia; or 9) life-threatening visceral disease or other severe concurrent diseases.
Estrogen, progesterone, and HER2/neu receptor status in tumors were obtained from patient histories when available. HER2/neu receptor status could be determined by immunohistochemical analysis or fluorescence in situ hybridization.
Safety was assessed via adverse events (AEs), vital signs, electrocardiogram, and laboratory tests. The investigator determined AE seriousness, severity grade, and relationship to study treatment. AEs were graded by the NCICTC for Adverse Events version 4.0.
Tumor response was assessed using RECIST Version 1.1. Magnetic resonance imaging or computed tomography scans were performed at screening, 8 weeks after the first dose of study treatment, and every 8 weeks after that until documented radiographic progression, the initiation of other anticancer therapy or death. The number of patients with complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were determined.
Pharmacokinetic sampling and analysis
For exemestane, plasma samples were collected at pre-dose and 1, 2, 4, 8, 12 and 24 h post-dose on d 1 of the run-in period and d 1 of combination treatment, respectively. For chidamide, plasma samples were collected at pre-dose and 1, 2, 4, 8, 12, 24, 48 h and 72 h post-dose on d 2 of the run-in period and d 1 of combination treatment, respectively. All plasma samples were stored at −10 °C to −30 °C until analysis.
A validated high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method was used to determine the plasma concentrations of chidamide (18,19) by Covance Analytical Laboratory (Shanghai, China). Plasma concentrations of exemestane were determined by Covance Laboratory from its internal validated method. The calibration curve range for chidamide and exemestane was 1−500 ng/mL and 0.2−100 ng/mL, respectively.
Statistical analysis
Descriptive statistics, such as mean, median, frequency and proportion, were used to summarize the results along with 95% confidence intervals (95% CI) where applicable. The Kaplan-Meier method was used to estimate PFS statistics. No inferential statistical analyses were performed due to its nature as a single-arm trial. All statistical analyses were performed using SAS software (Version 9.2; SAS Institute Inc., Cary, NC, USA).
The maximum plasma drug concentration (Cmax) and time to reach maximum concentration (Tmax) were obtained from experimental observation. Other PK parameters, including t1/2, area under the curve (AUC)0−t, AUC0−∞, clearance (CL/F), volume of distribution (Vd/F), and mean residence time (MRT), were calculated by a non-compartmental model with WinNonlin v6.4 (Pharsight Corp., Mountain View, CA, USA).
Results
Patient characteristics and disposition
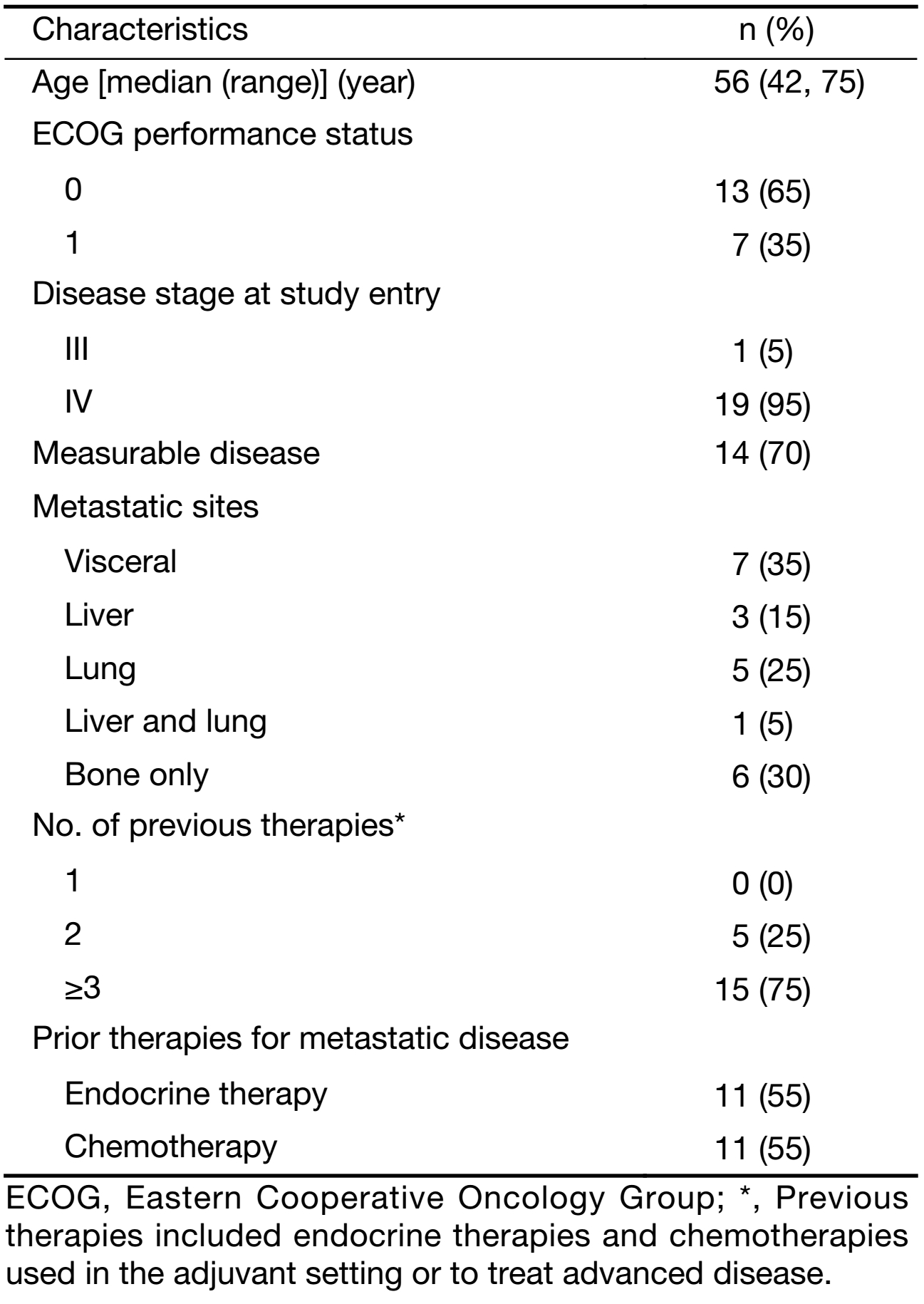
Between July 2015 and December 2015, 20 eligible patients were recruited, and their baseline characteristics are listed in Table 1. Most patients were in late middle age, and the median age was 56 years old. In this study, 35% of the patients had visceral involvement, and 30% had bone-only metastasis; 70% of the patients had measurable disease, and all other patients had at least one mainly lytic bone lesion. All patients had ER-positive tumors. The majority of patients (75%) had received ≥3 previous therapies before the study entry, and 55% of patients had received at least one salvage endocrine therapy and/or salvage chemotherapy.
Full table
The median number of treatment cycle was 5.2 (20.8 weeks). Two patients were still on treatment at the data cut-off date (October 17, 2017), with the treatment duration of 22 and 25 months, respectively. Three patients experienced at least one dose reduction of chidamide.
Safety
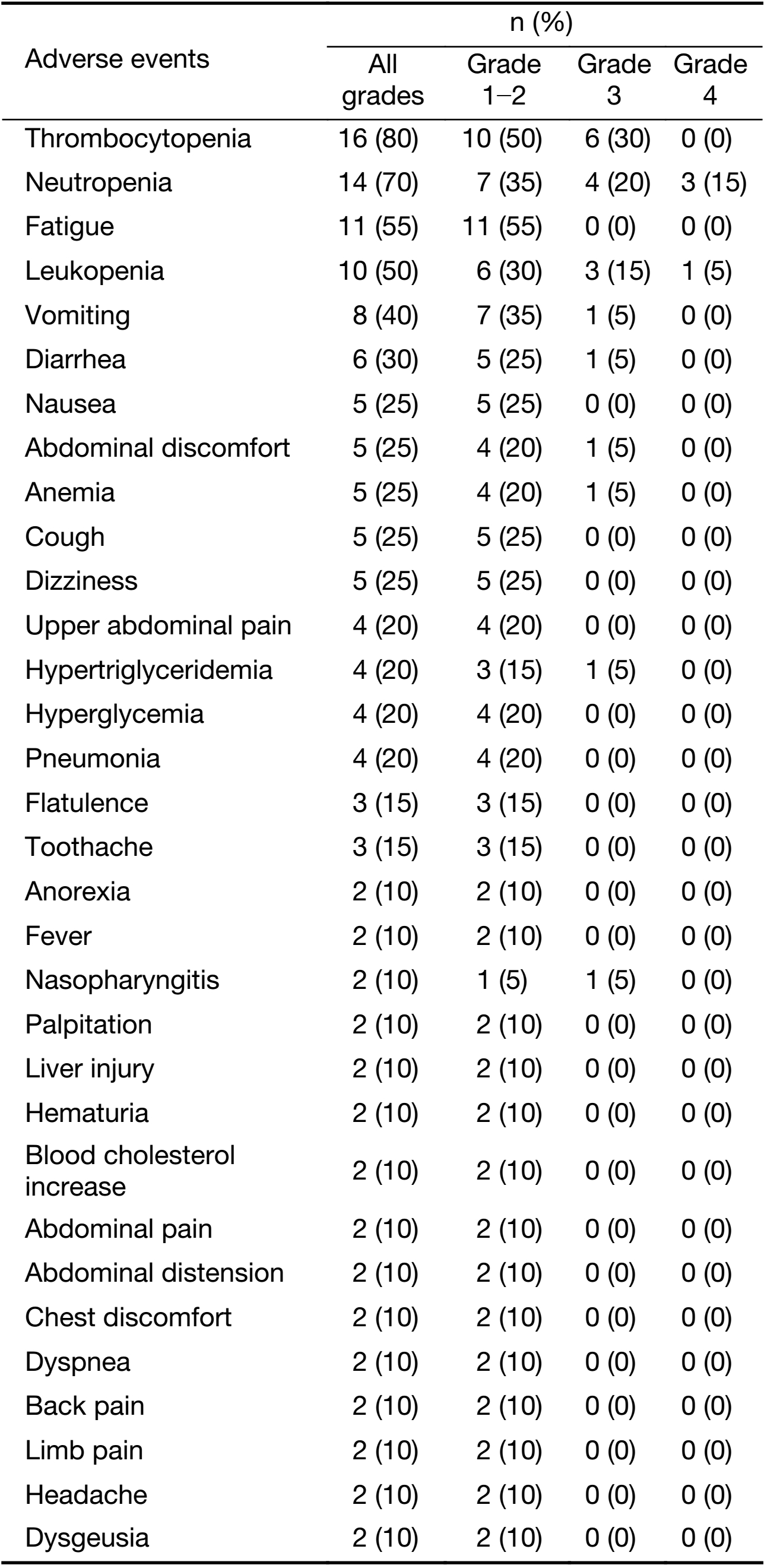
All 20 patients were assessed for toxicity. The most common AEs of the combination treatment (all grades) were thrombocytopenia (80%), neutropenia (70%), fatigue (55%), leucopenia (50%), vomiting (40%), diarrhea (30%), nausea (25%) and anemia (25%) (Table 2). The treatment-related grade 3−4 AEs in more than 2 patients were neutropenia (35%), thrombocytopenia (30%) and leucopenia (20%). Three patients discontinued due to AEs.
Full table
Four serious adverse events (SAE) were reported in 4 patients. Three SAEs were considered to be related to chidamide, including one grade 3 gastrointestinal dysfunction leading to hospitalization, one grade 2 myelosuppression with poor overall conditions leading to hospitalization, and one grade 3 thrombocytopenia leading to hospitalization. The symptoms resolved after temporary treatment discontinuation or dose reduction of chidamide, and the treatment continued for two patients who had been hospitalized with myelosuppression. No treatment-related death was reported.
PK
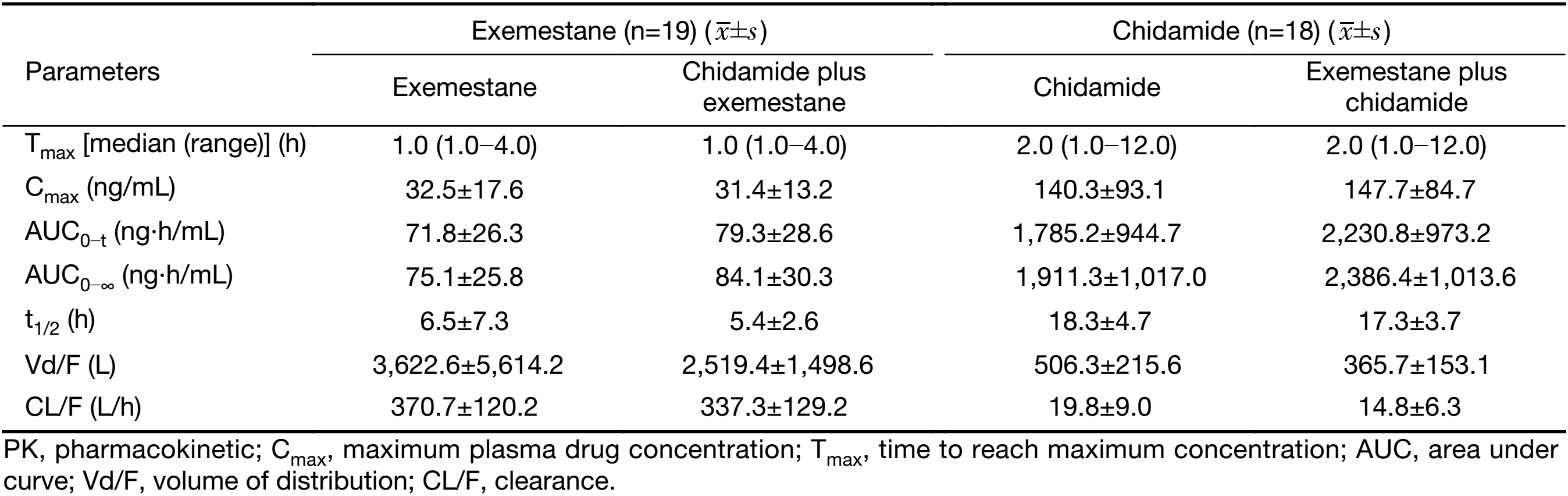
Ninteen patients completed the full collection of plasma samples for PK analysis. The PK parameters and the curve of mean plasma concentrations over time of either exemestane or chidamide alone and in combination are shown in Table 3 and Figure 1. There was no significant difference in PK parameters of exemestane before and after combination with chidamide, including the plasma exposure (32.5 vs. 31.4 ng/mL for Cmax, and 71.8 vs. 79.3 ng·h/mL for AUC0−t). For chidamide, while most PK parameters were similar between chidamide alone and combination with exemestane, a higher AUC value was noted after combination with exemestane (1,911.3 vs. 2,386.4 ng·h/mL). Inter and intrapatient variations were observed in chidamide PK parameters.
Full table
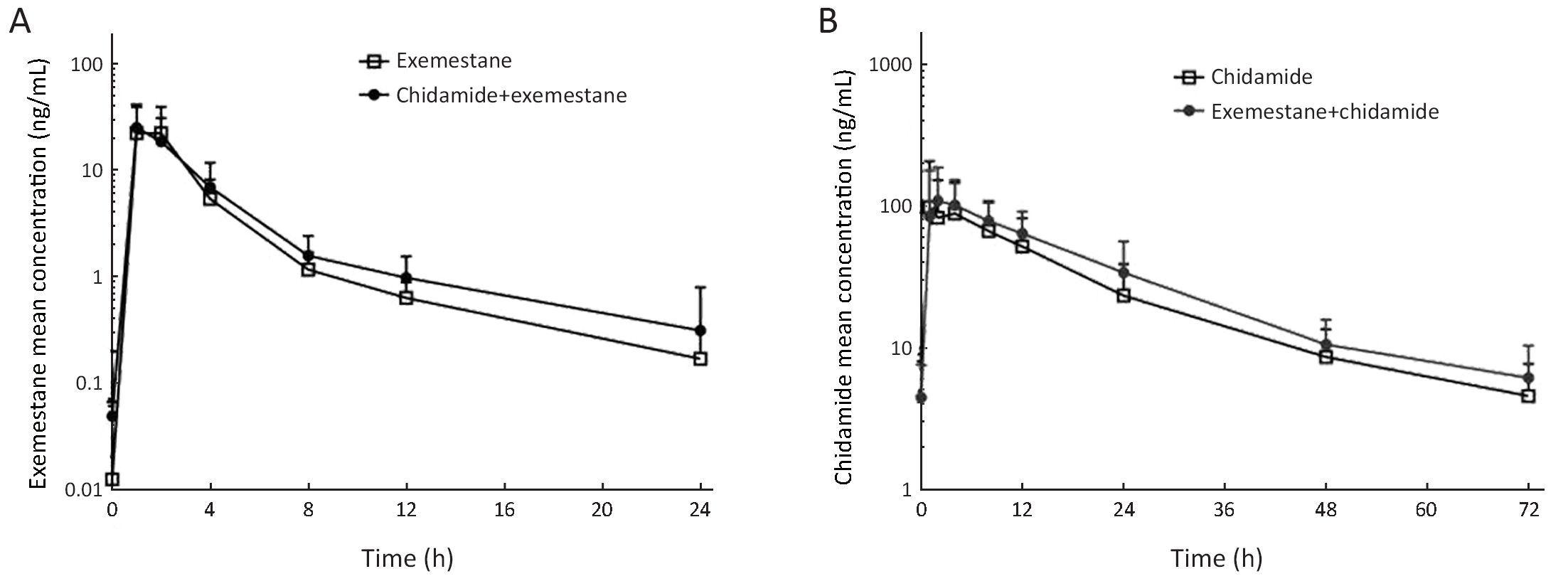
Preliminary efficacy
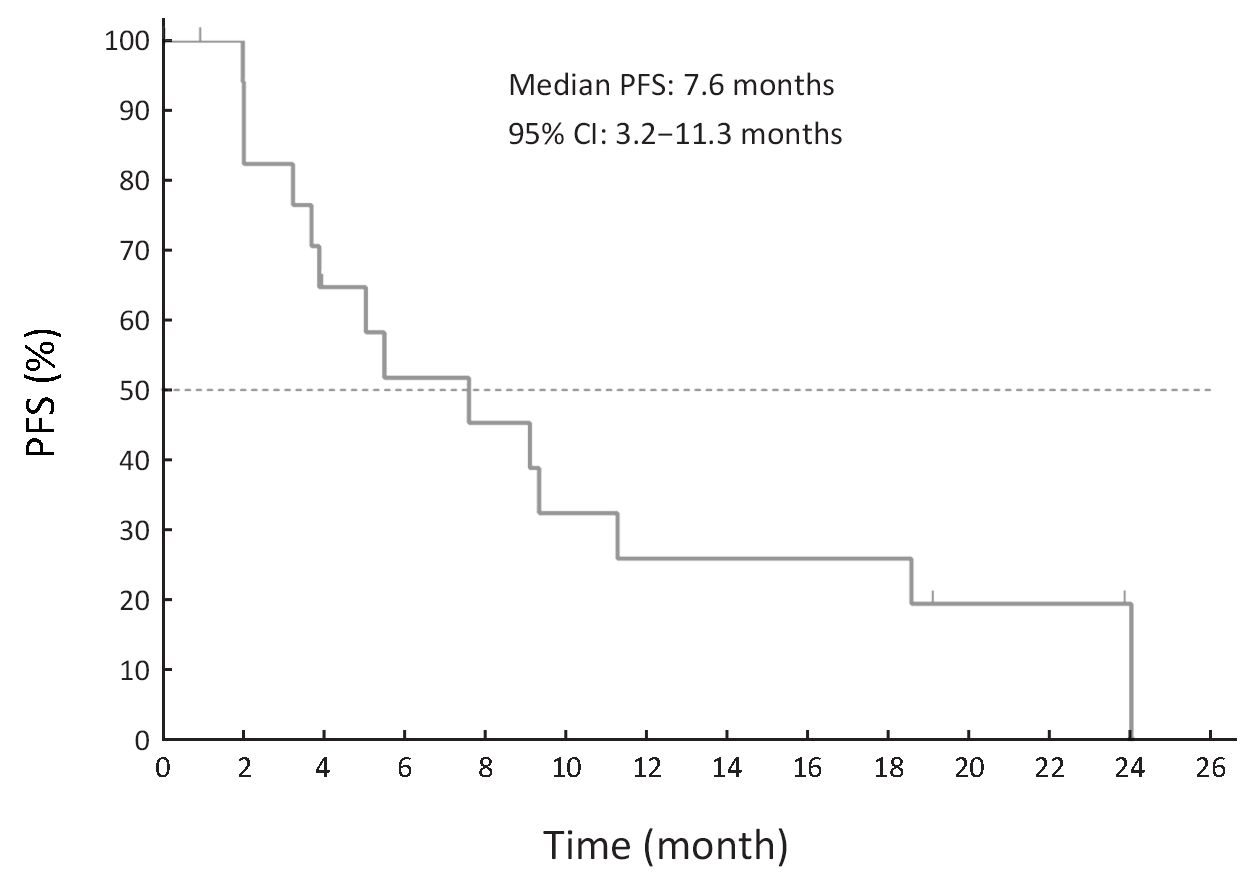
Best response in 16 of 20 patients (4 patients not evaluable) was assessed by RECIST, including 4 patients with PR, 10 patients with SD, and 2 patients with PD. Of the 14 patients with measurable disease, 4 had PR, 5 had SD, and 2 had PD (Table 4). Median PFS was 7.6 months (231 days) (Figure 2).
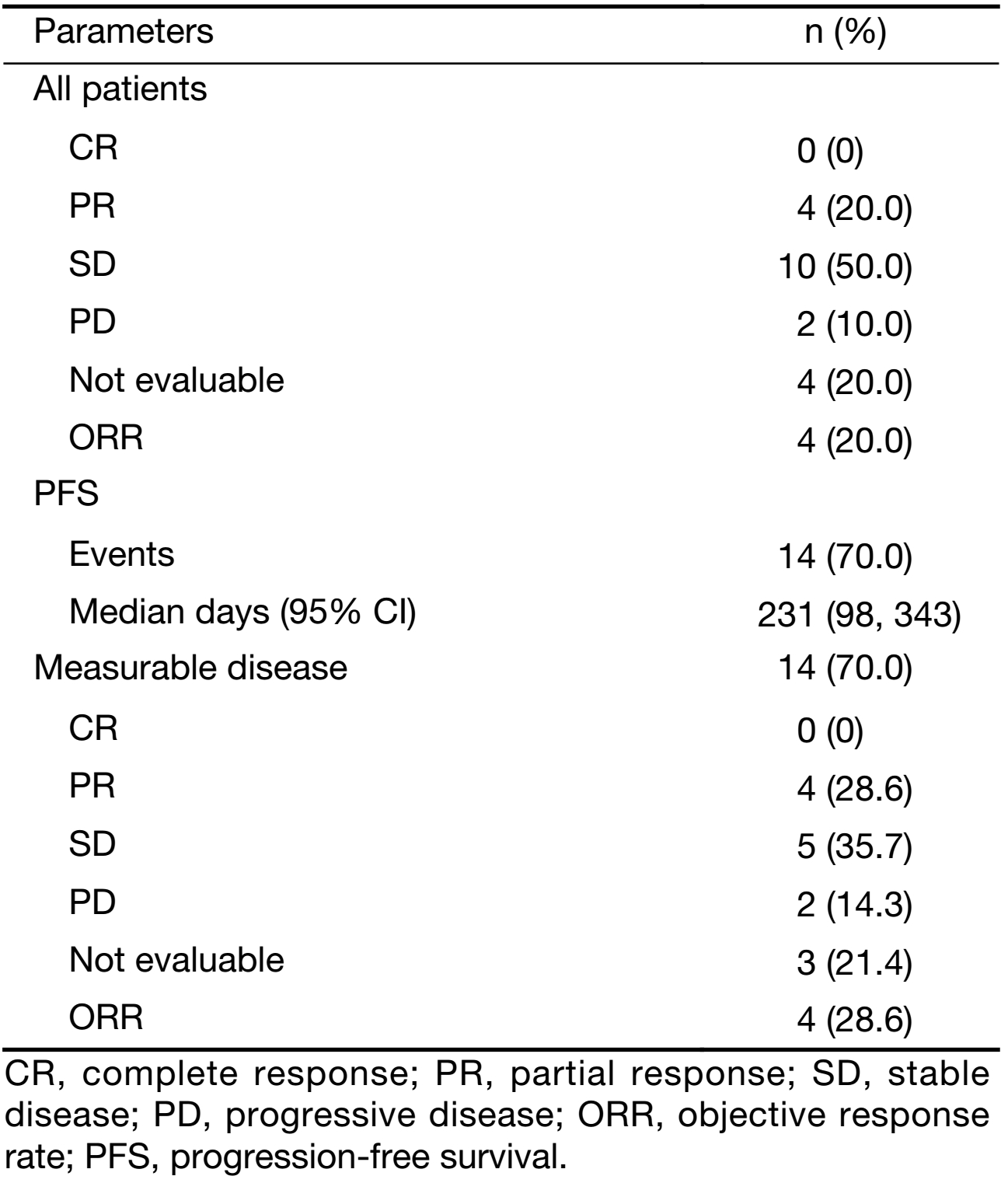
Full table
Discussion
Primary and secondary drug resistance is one of the major challenges in the treatment of HR+ breast cancer patients with endocrine therapies. Chidamide, functioning as a genuine epigenetic modulator, affects multiple cellular signaling pathways that are abnormally changed in cancer by selective inhibition of HDAC 1, 2, 3 and 10 (13), including the reversal of endocrine resistance in ER-positive breast cancer cells in vitro (17). Several HDAC inhibitors combined with endocrine drugs, including vorinostat and tamoxifen (20), panobinostat and letrozole (21), and entinostat and exemestane (10), have shown the encouraging efficacy in clinical trials in ABC patients. In this exploratory clinical study, we evaluated the safety, PK, and preliminary efficacy of chidamide in combination with exemestane in Chinese patients with HR+ ABC progressed on previous endocrine therapy.
Chidamide 30 mg BIW was the dosing regimen approved for the PTCL indication, which had been shown significant single-agent activity and a manageable safety profile either from the pivotal clinical trial or postmarketing real-world study in relapsed or refractory PTCL patients (12,22). Based on the known PK and safety profiles from each chidamide and exemestane, we employed 30 mg chidamide as the starting dose and kept the opportunity to escalate the chidamide dose down if the potential tolerance and/or drug-drug interaction issues aroused. The results from the study showed that the combination therapy of chidamide 30 mg BIW and exemestane 25 mg daily was generally well tolerated. The toxicity profile was similar to that previously observed from chidamide monotherapy (12,22). The most frequently reported AEs were hematological and gastrointestinal toxicities, including thrombocytopenia, neutropenia, nausea, vomiting and diarrhea. The more frequent grade 3−4 AEs included neutropenia (35%), thrombocytopenia (30%) and leucopenia (20%). The more common hematological AEs were also reported for exemestane monotherapy in ABC patients (23). The apparently higher grade of hematological AEs observed in the current study compared with chidamide-monotherapy in PTCL patients (12), might be due to the overlapping toxicities from each drug in the combination treatment. Most hematological AEs were recovered or relieved after dose reduction or temporary discontinuation of chidamide, and none of the patients withdrew the study due to those AEs.
Previous clinical study of chidamide in combination with paclitaxel and carboplatin in patients with non-small cell lung cancer has shown no evidence that chemotherapy drugs significantly affect PK behaviors of chidamide (19). The overall PK results from this study indicate that combined application of exemestane and chidamide does not affect the PK parameters of each other to a significant degree. While there was little difference in PK behaviors for exemestane before and after combination usage with chidamide, a potential higher blood exposure of chidamide was noted together with exemestane, which was probably due to the inter- and intra-patient variations for chidamide as reported previously (24).
Several exploratory clinical studies have demonstrated promising efficacy results in HR+ ABC patients by combination treatment of HDAC inhibitors and hormone therapies. For example, in a randomized phase II trial, while entinostat in combination with exemestane compared with placebo with exemestane, did not reach PFS primary endpoint (4.3 vs. 2.3 months; P=0.11) and the ORR was similar between the two groups (6.3% vs. 4.6%), the median OS was significantly prolonged in the entinostat group (28.1 vs. 19.8 months; P=0.036) (10). Our results from the current study have shown that combination treatment of ABC patients progressed on previous endocrine therapy with chidamide and exemestane appears to have higher ORR (20%) and longer median PFS (7.6 months) compared with the data from a similar patient population for entinostat + exemestane, as well as exemestane alone (10).
Conclusions
The results of this study show that the combination regimen of chidamide plus exemestane is generally well tolerable with promising preliminary efficacy in HR+ ABC patients progressed on previous endocrine therapy, which encourages further pivotal clinical trial in this patient population.
Acknowledgements
The authors thank all the patients, physicians and nurses who participated in this study. This study was partly supported by grants from the Chinese National Major Project for New Drug Innovation (No. 2015ZX09101005) and the Shenzhen municipal science and technology project (No. JCYJ20120618162903087 and No. JSGG20140515161400837).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Anderson WF, Chatterjee N, Ershler WB, et al. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat 2002;76:27–36. [PubMed] DOI:10.1023/A:1020299707510
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84. [PubMed] DOI:10.1016/S0140-6736(11)60993-8
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233–47. DOI:10.1146/annurev-med-070909-182917
- DeMichele A, Chodosh LA. " Braking” the cycle of resistance in endocrine therapy for breast cancer. Clin Cancer Res 2015;21:4999–5001. [PubMed] DOI:10.1158/1078-0432.CCR-15-1146
- Falahi F, van Kruchten M, Martinet N, et al. Current and upcoming approaches to exploit the reversibility of epigenetic mutations in breast cancer. Breast Cancer Res 2014;16:412. [PubMed] DOI:10.1186/s13058-014-0412-z
- Li Y, Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med 2016;6:pii: a026831. DOI:10.1101/cshperspect.a026831
- Sharma D, Saxena NK, Davidson NE, et al. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res 2006;66:6370–8. [PubMed] DOI:10.1158/0008-5472.CAN-06-0402
- Sabnis GJ, Goloubeva O, Chumsri S, et al. Functional activation of the estrogen receptor-α and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res 2011;71:1893–903. [PubMed] DOI:10.1158/0008-5472.CAN-10-2458
- Sabnis GJ, Goloubeva OG, Kazi AA, et al. HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2. Mol Cancer Ther 2013;12:2804–16. [PubMed] DOI:10.1158/1535-7163.MCT-13-0345
- Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 2013;31:2128–35. [PubMed] DOI:10.1200/JCO.2012.43.7251
- Pan DS, Yang QJ, Fu X, et al. Discovery of an orally active subtype-selective HDAC Inhibitor, chidamide, as an epigenetic modulator for cancer treatment. Med. Chem. Commun 2014;5:1789–96. DOI:10.1039/C4MD00350K
- Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol 2015;26:1766–71. [PubMed] DOI:10.1093/annonc/mdv237
- Ning ZQ, Li ZB, Newman MJ, et al. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol 2012;69:901–9. [PubMed] DOI:10.1007/s00280-011-1766-x
- Yao Y, Zhou J, Wang L, et al. Increased PRAME-specific CTL killing of acute myeloid leukemia cells by either a novel histone deacetylase inhibitor chidamide alone or combined treatment with decitabine. PLoS One 2013;8:e70522. [PubMed] DOI:10.1371/journal.pone.0070522
- Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov 2014;13:673–91. [PubMed] DOI:10.1038/nrd4360
- Pili R, Quinn DI, Hammers HJ, et al. Immunomodulation by entinostat in renal cell carcinoma patients receiving high-dose interleukin 2: A multicenter, single-arm, phase I/II trial (NCI-CTEP#7870). Clin Cancer Res 2017;23:7199–208. [PubMed] DOI:10.1158/1078-0432.CCR-17-1178
- Zhou Y, Wang Y, Zhang K, et al. Reverse effect of chidamide on endocrine resistance in estrogen receptor-positive breast cancer. Journal of Shenzhen University Science and Engineering (in Chinese) 2018;35:339–44. DOI:10.3724/SP.J.1249.2018.04000
- Hertz DL, Kidwell KM, Seewald NJ, et al. Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenomics J 2017;17:521–7. [PubMed] DOI:10.1038/tpj.2016.60
- Hu X, Wang L, Lin L, et al. A phase I trial of an oral subtype-selective histone deacetylase inhibitor, chidamide, in combination with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer. Chin J Cancer Res 2016;28:444–51. [PubMed] DOI:10.21147/j.issn.1000-9604.2016.04.08
- Munster PN, Troso-Sandoval T, Rosen N, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res 2001;61:8492–7. [PubMed]
- Tan WW, Allred JB, Moreno-Aspitia A, et al. Phase I study of panobinostat (LBH589) and letrozole in postmenopausal metastatic breast cancer patients. Clin Breast Cancer 2016;16:82–6. [PubMed] DOI:10.1016/j.clbc.2015.11.003
- Shi Y, Jia B, Xu W, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol 2017;10:69. [PubMed] DOI:10.1186/s13045-017-0439-6
- Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 2008;26:4883–90. [PubMed] DOI:10.1200/JCO.2007.14.4659
- Dong M, Ning ZQ, Xing PY, et al. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother Pharmacol 2012;69:1413–22. [PubMed] DOI:10.1007/s00280-012-1847-5