Construction and external validation of a nomogram that predicts lymph node metastasis in early gastric cancer patients using preoperative parameters
Introduction
According to the Japanese Gastric Cancer Association, early gastric cancer (EGC) is defined by lesions in the stomach that are confined to the mucosa and/or submucosa, regardless of size or lymph node metastasis (LNM) status (1). The curative treatment is radical gastrectomy with regional lymph node dissection because lymph node metastases are found in 2% to 18% of EGC patients (2,3). EGC has a much better prognosis than advanced stages of gastric cancer, and the 5-year survival rate exceeds 95% with proper treatment (4). Previous studies have shown that regional LNM is the most significant prognostic factor in EGC (5); thus, it is the most important consideration when treating EGC patients.
Tumor size, histologic type, the depth of invasion, the presence of an ulcer, and lymphatic invasion are risk factors for LNM in EGC as reported in previous studies (6). However, most of these studies were based on postoperative findings, especially pathology results, which are not available when attempting to predict the likelihood of LNM before surgery. With the development and universal use of preoperative examination methods, such as gastroscopy, endoscopic ultrasonography (EUS) and computed tomography (CT), surgeons may be able to obtain additional data on the lesion and regional lymph node status before surgery. A nomogram is a device or model that uses an algorithm or mathematical formula composed of several variables to predict the probability of an event or outcome (7), and such tools have been used in several types of cancer, such as breast cancer and prostate cancer (8,9). This study focuses on preoperative clinical data and examination results and their relationship to LNM in EGC to develop a predictive nomogram.
Materials and methods
Patients
We retrospectively analyzed 272 patients at Peking University Cancer Hospital between November 2010 and November 2015 as the primary cohort for the construction of a nomogram. Eligible patients were: being treated for the first time, underwent radical gastrectomy plus standard D1+/D2 lymph node dissection, had pathologically confirmed adenocarcinoma and EGC, and underwent complete preoperative examinations, including gastroscopy, EUS, CT and biopsy during gastroscopy. The exclusion criteria were as follows: 1) patients with neoadjuvant therapy; 2) previous history of cancer; 3) two or more sites of primary gastric cancer; 4) Siewert type I gastroesophageal junction carcinoma; or 5) distant metastasis. A cohort of 81 patients was recruited from December 2015 to July 2016 using the same inclusion and exclusion criteria as the primary cohort and prospectively analyzed to validate the nomogram. This study was approved by the Institutional Review Board of the Beijing Cancer Hospital, and informed consent was obtained from all individuals.
Parameters
The clinical characteristics of the patients, including gender and age, were collected. Gastroscopy data were gathered from the report. These data included the location of the lesion, the size of the tumor, the macroscopic type and the presence of ulcers. The location of the tumor was described in both the vertical and the horizontal planes. In the vertical plane, tumors were classified according to their location in the esophagogastric junction (EGJ) or upper (U), middle (M), or lower (L) portion of the stomach. In the cross-sectional plane, tumors were classified according to their location in the lesser (Less) or greater (Gre) curvature and the anterior (Ant) or posterior (Post) wall, as outlined in the Japanese classification of gastric carcinoma (3rd edition). The maximum diameter of the lesion measured during gastroscopy was recorded as the size of the tumor on endoscopy. The patients were categorized into two groups based on the macroscopic appearance of their tumors: those with non-depressed tumors and those with depressed and advanced tumors. Non-depressed tumors were those that were classified as protruding (I), superficially elevated (IIa), flat (IIb), or superficially depressed (IIc). The depressed and advanced tumor group included those with depressed-type tumors (III) and advanced gastric cancer; certain EGCs were macroscopically similar to advanced gastric cancer. The presence of an ulcer was defined by lesions with ulceration or scarring from previous ulceration (converging folds or deformity of the muscularis propria or fibrosis in the submucosal or deeper layer). The EUS parameters included the maximum diameter of the lesion, the depth or thickness of the tumor, the presence of metastatic lymph nodes (N stage), and in cases of LNM, the maximum diameter of the lymph nodes. The CT parameters, such as the vertical location of the tumor (EGJ, U, M, L), the thickness of the lesion, the presence and maximum diameter of enlarged lymph nodes, and the presence of ulceration were gathered from the reports and confirmed by two independent radiologists. All the patients had pathologically confirmed gastric cancer based on biopsy, and the cancer was categorized into differentiated (well and moderately differentiated tubular adenocarcinomas and papillary adenocarcinomas) or undifferentiated types (poorly differentiated adenocarcinomas and signet-ring cell carcinomas). All these parameters were defined in accordance with the Japanese classification of gastric carcinoma (3rd edition).
Statistical analysis
The statistical analyses were performed and graphs were constructed using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA) and R software (Version 3.2.1; R Foundation for Statistical Computing, Vienna, Austria). For continuous variables, a test of normality was first performed. For normally distributed variables, differences between groups were analyzed using Student’s t test, and for non-normally distributed variables, the rank sum test was used. The Chi-squared test and Fisher’s exact test (when appropriate) were used for comparisons of categorical variables. Significant factors noted on univariate analysis were subsequently entered into a multivariate logistic regression model for analysis. Two-sided P<0.05 were considered statistically significant. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to assess the predictive accuracy of the logistic model. Meaningful predictive factors from the multivariate logistic regression were used to formulate a nomogram to estimate the risk of LNM in EGC. The internal validation of the nomogram included two steps. First, a concordance index (c-index) was calculated using the bootstrap resampling method to assess the discriminative ability of the nomogram; the c-index measures the model’s ability to differentiate patients with different outcomes, which is similar to the AUC of a ROC curve. The second step was calibration, which was performed by reviewing plots of predicted probabilities from the nomogram versus the actual probabilities. The ideal nomogram with 100% accuracy would be a graph in which the observed and predicted probabilities fall along the 45-degree line (10,11). In the external validation of the nomogram, the incidence of LNM for each of the 81 patients in the validation cohort was estimated based on the constructed nomogram, and another calibration curve was plotted to illustrate the actual predictive ability of the nomogram. The cut-off score of the nomogram was determined by the Youden index, and the sensitivity, specificity, positive predictive value, and negative predictive value of the nomogram were calculated for the two cohorts.
Results
A total of 272 patients in the primary cohort were involved in this study. The median age was 58 (range, 18−81) years and 182 (66.9%) of the patients were male. There were 37 (13.6%) patients pathologically confirmed to have LNM, including 22 (8.1%) patients with stage N1, 9 (3.3%) with stage N2 and 6 (2.2%) with stage N3 according to the Japanese classification of gastric carcinoma (3rd edition). The mean numbers of metastatic lymph nodes and the number of dissected lymph nodes were 3 (range, 1−13) and 25 (range, 8−64), respectively. Tumors were limited to the mucosal layer (T1a) in 128 (47.1%) patients, whereas the tumors invaded the submucosal layer (T1b) in 144 (52.9%) patients, and the percentages of lymph node positivity in these two groups of patients were 4.7% and 21.5%, respectively.
Detailed information on the distributions of categorical and continuous variables is shown in Tables 1, 2. The gastroscopy results showed that LNM was associated with circumferential lesions in the horizontal plane, a depressed and advanced macroscopic tumor type, the presence of an ulcer and the maximum diameter of the tumor. For the EUS parameters, the depth of invasion (T1b stage), N stage, maximum diameter, and thickness of the lesion, as well as the maximum diameter of the detected lymph nodes, were associated with LNM. The detection of enlarged lymph nodes on CT, the maximum diameter of these lymph nodes and the thickness of the lesion were risk factors for LNM in univariate analysis. The differentiated biopsy type obtained during gastroscopy was not associated with LNM.
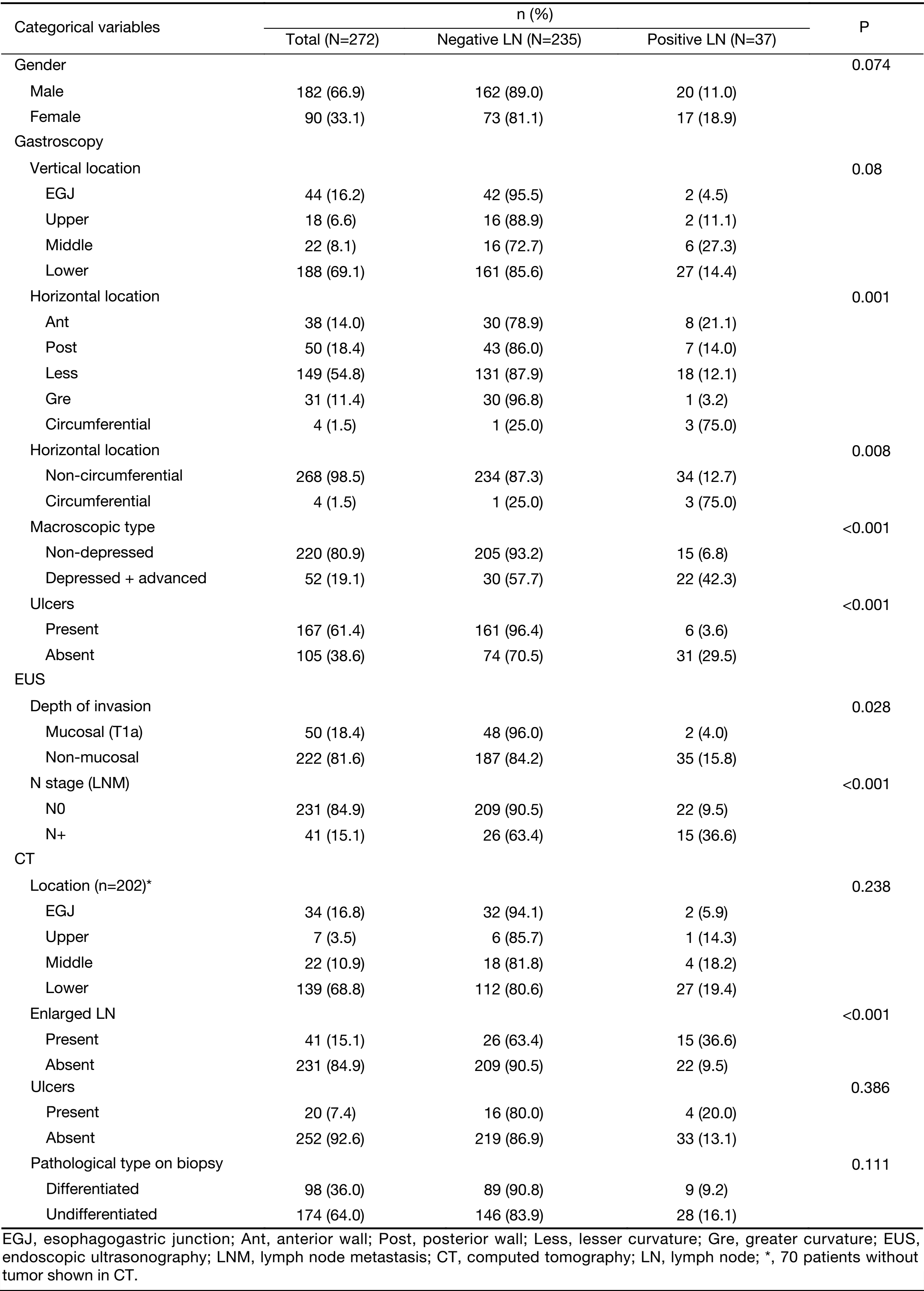
Full table
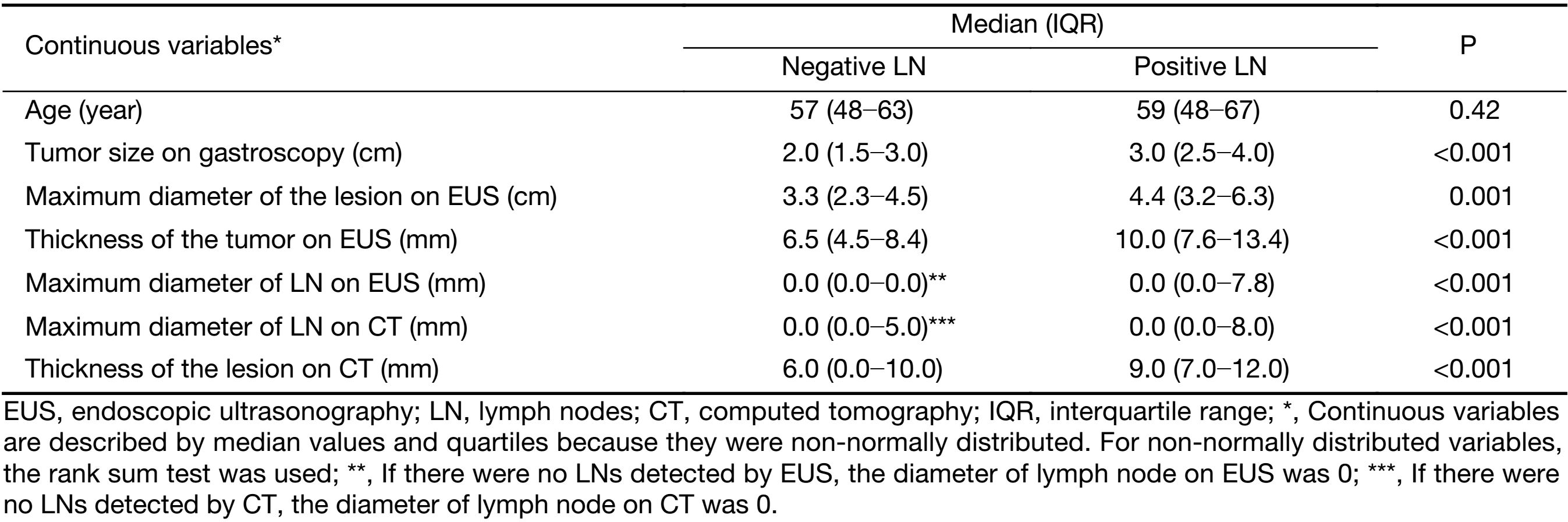
Full table
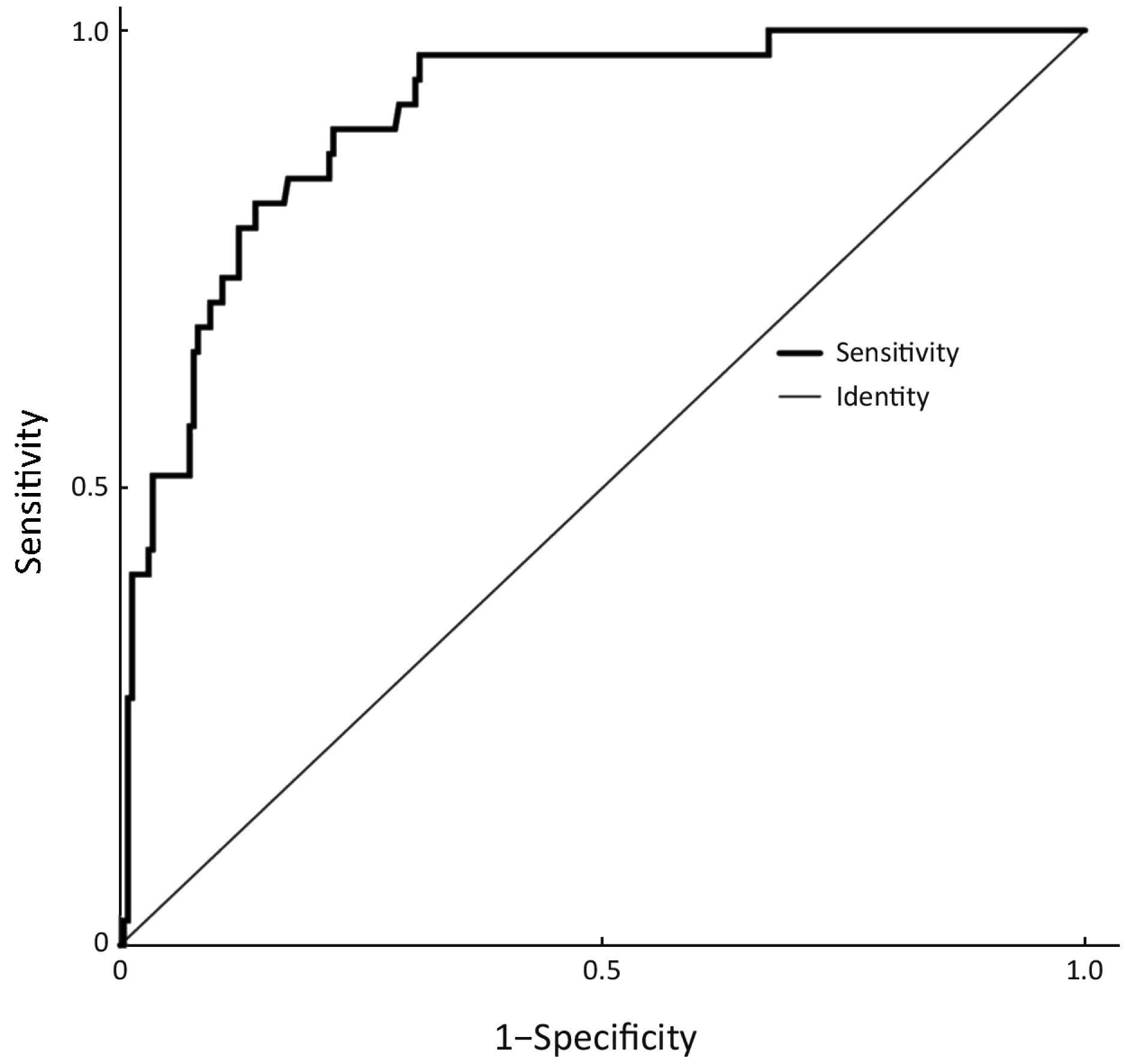
Parameters that were significant in the univariate analysis were included in the multivariate logistic regression analysis except for the horizontal tumor location on gastroscopy (circumferential lesion or not) because circumferential lesions were rarely observed in EGC and because the 3 suspected circumferential lesions in our sample population were eventually confirmed to be much smaller and confined to only one quarter of the stomach wall on postoperative pathology. The results of the multivariate logistic regression analysis are shown in Table 3. The presence of an ulcer (95% CI, 2.422−18.877) and the maximum diameter of the lesion (95% CI, 1.363−2.818) on gastroscopy, the thickness of the lesion on EUS (95% CI, 1.116−1.418) and the presence of enlarged lymph nodes on CT (95% CI, 1.129−6.657) were independent risk factors for LNM in EGC. An ROC curve was constructed to assess the predictive value of the regression model, which showed satisfactory predictive ability with an AUC of 0.905 (Figure 1).
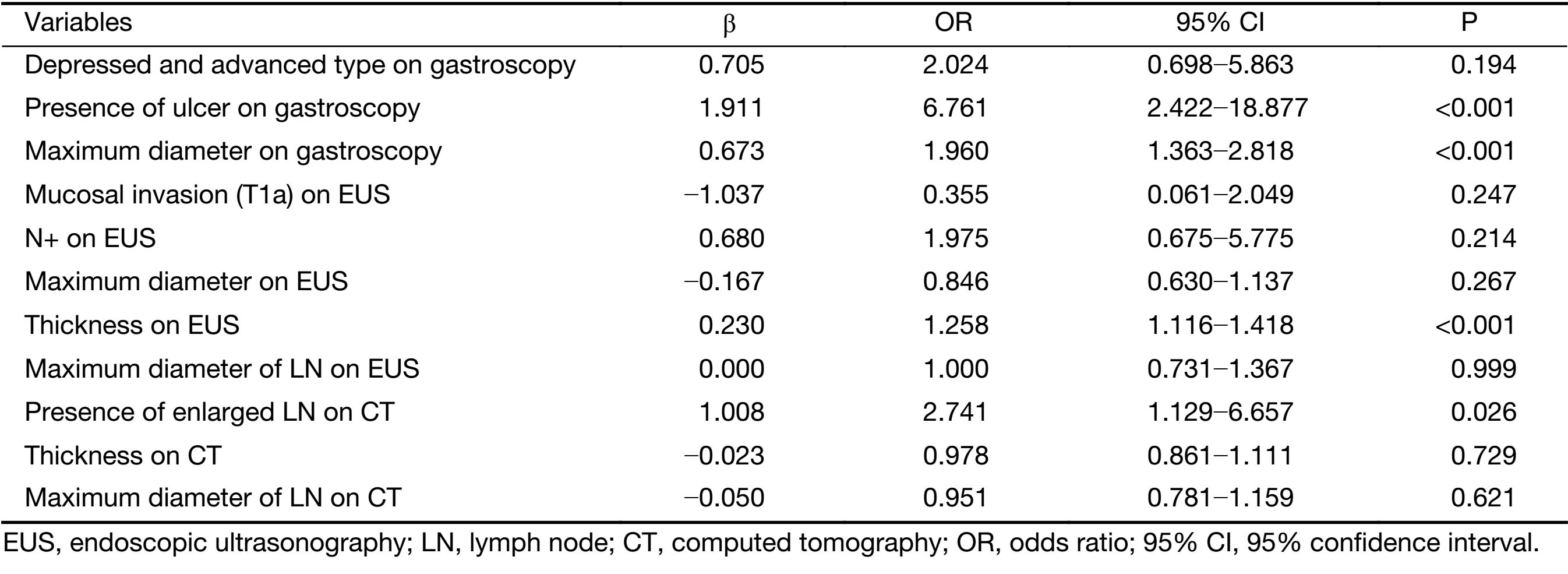
Full table
The four factors that were found to be significant in the multivariate analysis were used to construct the nomogram, as shown in Figure 2. The first row (“Points”) is used to assign a score for each variable below the first row by drawing a vertical line from the value for each variable to the “Points” line. The “Total points” are calculated by summing the scores for all the variables, and the final predicted risk of LNM for each patient is obtained by drawing a vertical line from the “Total points” line to the “Risk” line.
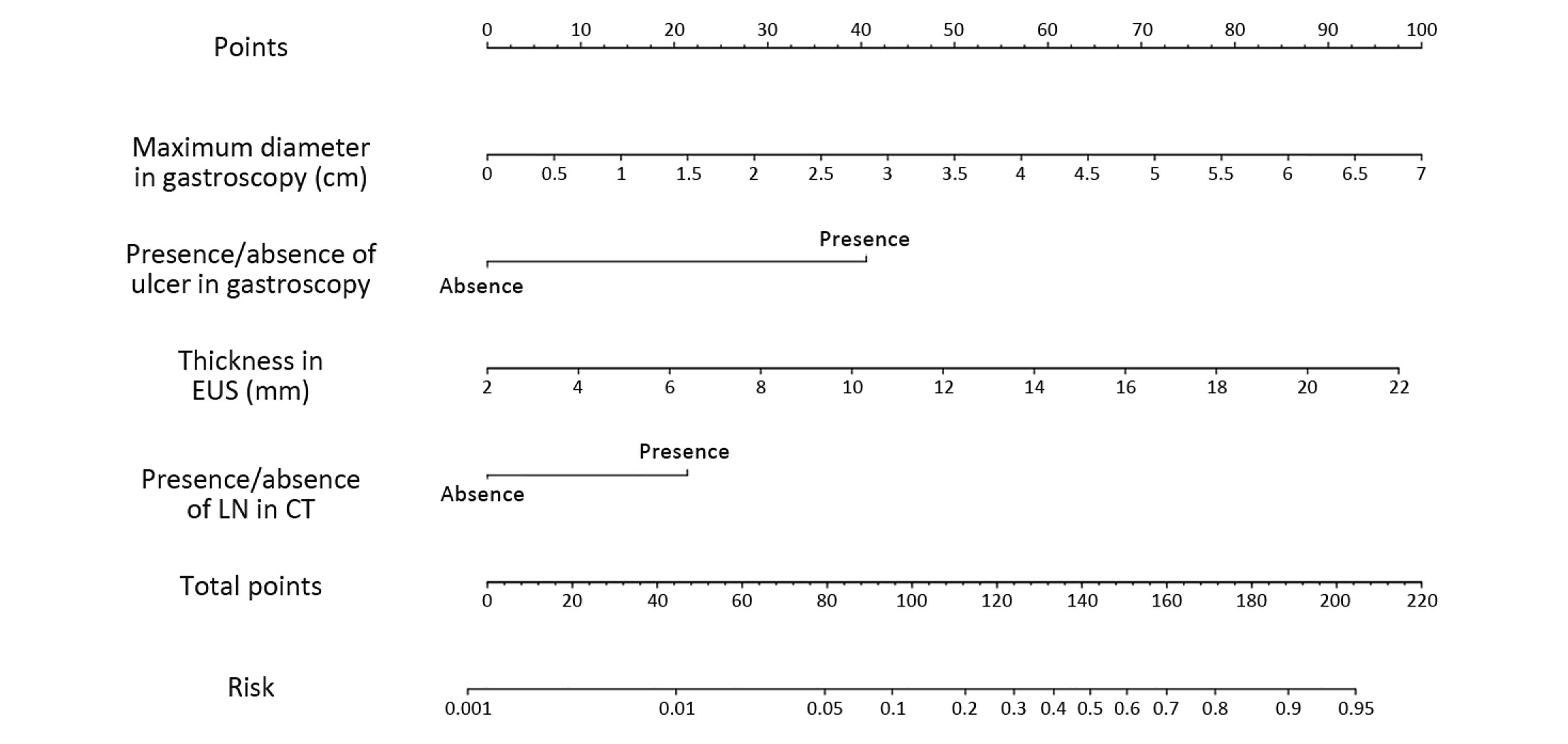
The cut-off score of the nomogram was 110, as determined by the Youden index. In the primary cohort, the sensitivity, specificity, positive predictive and negative predictive values of the nomogram were 81.1%, 86.0%, 47.6% and 96.7%, respectively.
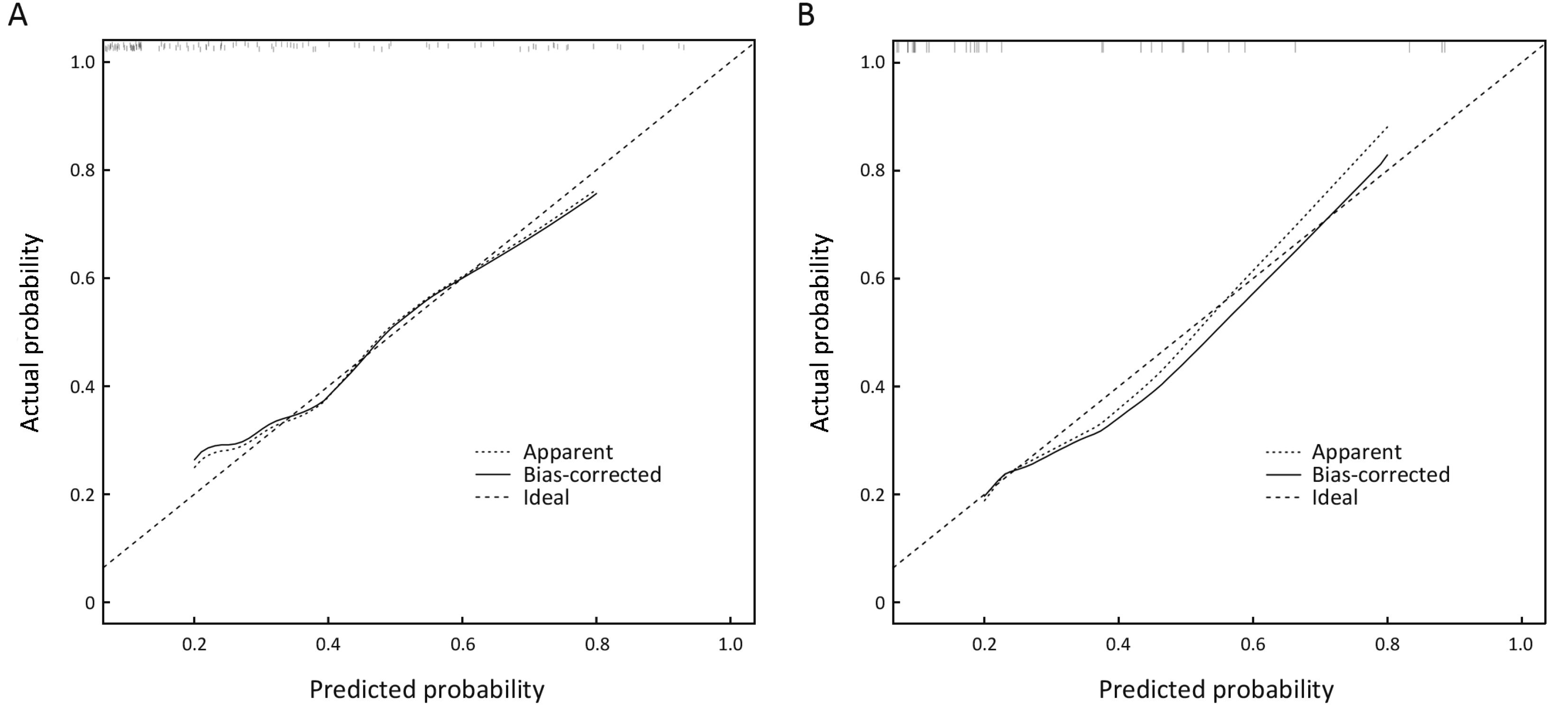
The internal validation of this nomogram involved two components. Discrimination was quantified with the c-index, and the value of 0.905 suggested that the constructed nomogram had a high accuracy in discriminating the patients’ lymph node status. The second component was calibration, which compared the predicted probability of LNM with the actual probability, as shown on the calibration curve in Figure 3. External validation was performed with the validation cohort. Another calibration curve was plotted for the validation cohort, and the sensitivity, specificity, positive predictive and negative predictive values of the nomogram in this cohort were 75.0%, 91.0%, 60.0% and 95.5% respectively.
Discussion
Gastric cancer is one of the most common cancers worldwide, especially in China, where over 423 thousand new cases were observed and nearly 300 thousand deaths were caused by gastric cancer in 2011 (12). EGC is defined as gastric cancer with tumor invasion limited to the mucosa or submucosa, regardless of the presence of LNM (3). Current treatment methods include minimally invasive endoscopic surgery, wedge resection, and gastrectomy with regional lymph node dissection laparoscopically or by open laparotomy (13). Endoscopic procedures, such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), allow for the resection of the lesion and preservation of stomach function but leave regional lymph nodes undissected and it should therefore only be conducted when the likelihood of LNM is extremely low and the site and size of the lesion are amenable to en bloc resection (14,15). Patients who underwent curative ESD as an absolute indication had a favorable 5-year survival that was not significantly different from that of patients who had laparoscopic or open surgery; thus, ESD could be employed as a standard treatment for EGC lesions in selected patients (16,17). For EGC patients, LNM is the most important prognostic factor, and predicting the risk of LNM is crucial when selecting treatment methods (18).
By retrospectively reviewing 3,131 ECG patients, Sekiguchi et al. demonstrated that tumor size, histological type, presence of ulcerative finding and presence of lymphovascular involvement were independent risk factors of LNM (19). Other studies also revealed that overexpression of CD44v6, increased tumor markers [carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, CA125], gender, age at diagnosis were also associated with LNM (20-23). However, most of these risk factors mentioned above were postoperative findings which were not available before treatment. Although some predictive models were constructed based on postoperative findings, the usefulness of these models were limited. In this research, the author only focused on the relationship of preoperative parameters and LNM in order to construct a true predictive model before treatment.
The gastric cancer guidelines of the National Comprehensive Cancer Network (NCCN) recommend upper gastrointestinal endoscopy with biopsy, abdomen and pelvis CT, and EUS as preoperative examinations for gastric cancer patients (24). However, none of these examinations alone could exactly estimate LNM. In this study, 2 of the 37 (5.4%) patients with positive lymph nodes met the criteria for ESD but were pathologically confirmed to have LNM, which indicated that endoscopy features alone were not reliable predicting LNM. As for CT, the presence and size of lymph nodes are measured routinely during CT scanning, but it is still difficult to distinguish metastatic lymph nodes because most metastatic lymph nodes are less than 10 mm, and only a small proportion of the patients with LNM exhibited an metastatic lymph node as the largest (25). In this study, 202 patients presented lymph nodes smaller than 5 mm, including 15 patients (7.4%) who had positive lymph nodes suggesting that preoperative CT scans alone might not be sufficiently accurate to predict lymph node status based on the presence or size of lymph nodes. EUS has been used for T staging of gastric cancer and is considered the best available method for the assessment of invasion depth which is one of the risk factors of LNM (26). However, in a Cochrane review, Mocellin et al. concluded that EUS was not sufficiently accurate to confirm or exclude the presence of LNM (27). Lee et al. also reported that the accuracy of EUS was similar to that of traditional endoscopy in the assessment of the depth of tumor invasion (73.6% and 66.7%, respectively), and proper treatment selection based on EUS occurred in 71.5% of cases, which is even lower than with endoscopy (75.3%) (28). In this study, the accuracy of EUS in the assessments of T1a status was 61.0% (166/272), which could explain the thickness of lesions rather than T1a/T1b was an independent risk factor of LNM. In previous studies, the differentiation type was associated with LNM in EGC, thus making it one of the criteria for ESD/EMR (29). However, Nakagawa et al. reported that preoperative histology was not a significant predictor of LNM for several reasons. One of the characteristics of gastric cancer is histological heterogeneity, and the limited amount of tissue collected by biopsy cannot always represent the dominant histology type of the tumor (30). The frequency of discrepancies in the histology between preoperative biopsy and postoperative pathology ranges from 16.3% to 53.7% (31,32). In this study, the accuracy of preoperative biopsy was 86.0%, which explained why the differentiation type on preoperative biopsy was not a significant predictor of LNM.
Nomogram is a graphic tool for individual probability of a clinical event based on a statistical predictive model. The advantage of nomogram is that it takes several risk factors into consideration when calculating the probability and it is practical because the nomogram directly presents the score and risk of certain clinical outcomes. Our study provides an effective tool for the selection of true lymph node-negative patients because of its high specificity and negative predictive value. Combining the nomogram presented here with other criteria such as the sentinel lymph node technique might represent a promising approach for predicting LNM and could provide the basis for organ-preserving gastrectomy, which is the next step of research in our center.
This research has certain limitations. It is an investigational study to propose a potential formula for predicting LNM in EGC. More efforts should be done before clinical application. The nomogram was constructed using a retrospective cohort, and systemic bias was inevitable because many of the patients with mucosal tumors underwent endoscopic surgery and were not included in the study, thus making the proportion of T1a tumors lower than that in the general population.
Conclusions
Our study revealed that the presence of ulceration and the maximum lesion diameter on gastroscopy, the thickness of the lesion on EUS and the presence of enlarged lymph nodes on CT were independent risk factors for LNM in EGC. A nomogram that can estimate the risk of LNM for EGC patients was created based on these risk factors. Patients who had a nomogram score of less than or greater than 110 were considered to have a low or high risk for LNM, respectively. This nomogram should provide more accurate information for surgeons treating EGC patients in their clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Saragoni L, Morgagni P, Gardini A, et al. Early gastric cancer: diagnosis, staging, and clinical impact. Evaluation of 530 patients. New elements for an updated definition and classification. Gastric Cancer 2013;16:549–54. [PubMed] DOI:10.1007/s10120-013-0233-2
- Espinel J, Pinedo E, Ojeda V, er al. Treatment modalities for early gastric cancer. World J Gastrointest Endosc 2015;7:1062–9. [PubMed] DOI:10.4253/wjge.v7.i12.1062
- Sun K, Chen S, Ye J, et al. Endoscopic resection versus surgery for early gastric cancer: a systematic review and meta-analysis. Dig Endosc 2016;28:513–25. [PubMed] DOI:10.1111/den.12596
- Wang H, Zhang H, Wang C, et al. Expanded endoscopic therapy criteria should be cautiously used in intramucosal gastric cancer. Chin J Cancer Res 2016;28:348–54. [PubMed] DOI:10.21147/j.issn.1000-9604.2016.03.09
- Suh DD, Oh ST, Yook JH, et al. Differences in the prognosis of early gastric cancer according to sex and age. Therap Adv Gastroenterol 2017;10:219–29. [PubMed] DOI:10.1177/1756283X16681709
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219–25. [PubMed] DOI:10.1007/PL00011720
- Silva TB, Oliveira CZ, Faria EF, et al. Development and validation of a nomogram to estimate the risk of prostate cancer in Brazil. Anticancer Res 2015;35:2881–6. [PubMed]
- van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 2003;10:1140–51. [PubMed] DOI:10.1245/ASO.2003.03.015
- Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol 2012;61:480–7. [PubMed] DOI:10.1016/j.eururo.2011.10.044
- Brennan MF, Kattan MW, Klimstra D, et al. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg 2004;240:293–8. [PubMed] DOI:10.1097/01.sla.0000133125.85489.07
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364–70. [PubMed] DOI:10.1200/JCO.2007.12.9791
- Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1–11. [PubMed] DOI:10.3978/j.issn.1000-9604.2016.02.08
- Zheng Z, Zhang Y, Zhang L, et al. Nomogram for predicting lymph node metastasis rate of submucosal gastric cancer by analyzing clinicopathological characteristics associated with lymph node metastasis. Chin J Cancer Res 2015;27:572–9. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.12.06
- Ishikawa S, Togashi A, Inoue M, et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer 2007;10:35–8. [PubMed] DOI:10.1007/s10120-006-0407-2
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3–15. [PubMed] DOI:10.1111/den.12518
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016;19:198–205. [PubMed] DOI:10.1007/s10120-015-0469-0
- Cho JH, Cha SW, Kim HG, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 2015;30:3762–73. [PubMed] DOI:10.1007/s00464-015-4672-1
- Lee T, Tanaka H, Ohira M, et al. Clinical impact of the extent of lymph node micrometastasis in undifferentiated-type early gastric cancer. Oncology 2014;86:244–52. [PubMed] DOI:10.1159/000358803
- Sekiguchi M, Oda I, Taniguchi H, et al. Risk stratification and predictive risk-scoring model for lymph node metastasis in early gastric cancer. J Gastroenterol 2016;51:961–70. [PubMed] DOI:10.1007/s00535-016-1180-6
- Eom BW, Joo J, Park B, et al. Nomogram incorporating CD44v6 and clinicopathological factors to predict lymph node metastasis for early gastric cancer. Plos One 2016;11:e0159424. [PubMed] DOI:10.1371/journal.pone.0159424
- Zhao LY, Yin Y, Li X, et al. A nomogram composed of clinicopathologic features and preoperative serum tumor markers to predict lymph node metastasis in early gastric cancer patients. Oncotarget 2016;7:59630–9. [PubMed] DOI:10.18632/oncotarget.10732
- Guo CG, Zhao DB, Liu Q, et al. A nomogram to predict lymph node metastasis in patients with early gastric cancer. Oncotarget 2017;8:12203–10. [PubMed] DOI:10.18632/oncotarget.14660
- Li X, Liu S, Yan J, et al. The characteristics, prognosis, and risk factors of lymph node metastasis in early gastric cancer. Gastroenterol Res Pract 2018;2018:6945743. [PubMed] DOI:10.1155/2018/6945743
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Gastric Cancer Version 2. 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Kim DJ, Kim W. Is lymph node size a reliable factor for estimating lymph node metastasis in early gastric cancer?. J Gastric Cancer 2018;18:20–9. [PubMed] DOI:10.5230/jgc.2018.18.e1
- Pei Q, Wang L, Pan J, et al. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J Gastroenterol Hepatol 2015;30:1566–73. [PubMed] DOI:10.1111/jgh.13014
- Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2015:CD009944. [PubMed] DOI:10.1002/14651858.CD009944.pub2
- Lee JY, Choi IJ, Kim CG, et al. Therapeutic decision-making using endoscopic ultrasonography in endoscopic treatment of early gastric cancer. Gut Liver 2016;10:42–50. [PubMed] DOI:10.5009/gnl14401
- Wang YW, Zhu ML, Wang RF, et al. Predictable factors for lymph node metastasis in early gastric cancer analysis of clinicopathologic factors and biological markers. Tumour Biol 2016;37:8567–78. [PubMed] DOI:10.1007/s13277-015-4721-3
- Nakagawa M, Choi YY, An JY, et al. Difficulty of predicting the presence of lymph node metastases in patients with clinical early stage gastric cancer: a case control study. BMC Cancer 2015;15:943. [PubMed] DOI:10.1186/s12885-015-1940-3
- Takao M, Kakushima N, Takizawa K, et al. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer 2012;15:91–6. [PubMed] DOI:10.1007/s10120-011-0075-8
- Min BH, Kang KJ, Lee JH, et al. Endoscopic resection for undifferentiated early gastric cancer: focusing on histologic discrepancies between forceps biopsy-based and endoscopic resection specimen-based diagnosis. Dig Dis Sci 2014;59:2536–43. [PubMed] DOI:10.1007/s10620-014-3196-1
