WWOX suppresses KLF5 expression and breast cancer cell growth
Introduction
Located at one of the most active common fragile sites involved in cancer, FRA16D, the WW domain-containing oxidoreductase (WWOX) has been reported to be a tumor suppressor. Likewise, loss of WWOX expression or function has been identified in a number of tumors, including breast and bladder cancer (1-3). Results from animal models revealed that Wwox–/– mice are postnatal lethal, and the Wwox+/– mice are more prone to develop spontaneous breast tumors as compared to their matched-littermate controls (1,4), supporting a tumor suppressing function of WWOX.
WWOX is a 46-KDa protein that contains two N-terminal WW domains and a central short-chain dehydrogenase/reductase (SDR) domain (5). A previously published study suggested that WWOX predominately functions through its first WW domain, which binds the proline-tyrosine rich PY motifs (PPxY, where P is proline, Y is a tyrosine and x is any amino acid) of a partner. WWOX has also been shown to regulate a number of cellular processes, including cell growth, differentiation, and apoptosis, through interacting with several proteins including p73 (6), Ap2α and γ (7), ErbB4 (8,9), etc. Despite these findings, the functional mechanisms of WWOX have not been fully explained to date.
The Krüppel-like transcription factor 5 (KLF5/IKLF/BTEB2) has been suggested as an oncogene in multiple carcinomas (10) and reported to promote cell proliferation (11-14), migration (15), and tumorigenesis (11) in different cell models, including bladder and breast cancer cells. Moreover, a high expression level of KLF5 was reported to be an unfavorable prognostic biomarker correlated with shorter survival among breast cancer patients (10,16).
According to the gene expression status of the estrogen receptor (ERα), progesterone receptor (PR) and human epidermal growth factor-2 (Her-2), molecule-based classification of breast cancers has defined several different subtypes: the luminal subtype (ER+ and/or PR+), the Her-2 subtype (Her-2+), and basal subtype (usually ER-/PR-/Her-2–) (17,18). The basal-like subtype of cancer is associated with more aggressive histology, poorer prognosis, more increased unresponsiveness to typical endocrine therapies, as well as shorter survival as compared with the other subtypes (19,20). Recently, WWOX expression was reported to be frequently reduced in basal-like breast cancers; similarly decreased WWOX expression is associated with the basal-like subtype and a poor disease-free survival rate for breast cancer patients (7,21). Intriguingly, KLF5 has been found to be overexpressed in basal-like breast cancers (14,16) and contains a PY motif. These reports prompted us to consider the connection between WWOX and KLF5, and in this study we demonstrate that WWOX interacts with KLF5 and suppresses the oncogenic functions of KLF5 in cancers.
Materials and methods
Cell culture and plasmids transfection
HCC1937 breast cancer cells were cultured in RPMI-1640 (with 2.05 mM L-glutamine) supplemented with 5% fetal bovine serum (FBS), 4.5 g/L glucose, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, and 1% penicillin/streptomycin (P/S). The TSU-Pr1 derived bladder cancer cell clone with stable KLF5 expression was maintained in RPMI-1640 (with 2.05 mM L-glutamine) supplemented with 5% FBS and 1% P/S. Human embryonic kidney 293T (HEK293T) cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM), containing 5% FBS, and 1% P/S.
All plasmid constructs were transfected to 293T cells using lipofectamine 2000 (Invitrogen, Carlsbad, USA) following the manufacturer’s protocols.
Western blotting assay and antibodies
Cell lysates were prepared as described previously (22), and protein concentration was measured using the protein assay kit (Bio-Rad, Hercules, CA, USA). We separated 40 µg protein samples by SDS-PAGE and blotted onto PVDF membranes. After incubating with a specific primary antibody and peroxidase-conjugated secondary antibody, signals were visualized using a Fujifilm LAS-3000 image system.
The anti-HA (Y-11) rabbit polyclonal (sc-805) antibody (Ab) was obtained from Santa Cruz Biotechnology (Santa Cruz, USA). The anti-GST rabbit monoclonal (G7781), anti-Flag (F3165) and anti-β-actin (AC-15) mouse monoclonal (A5441) Abs were from Sigma-Aldrich (St. Louis, USA). The anti-KLF5 rabbit polyclonal Ab has been described previously (22), and the anti-FGF-BP mouse antibody was purchased from R&D Systems (Minneapolis, MN, USA).
Establish of stable WWOX-inducible cells
The primers 5'-TTGGATCCATGGCAGCGCTGCGCTACGC-3' (forward, BamHI site is underlined) and 5'-ATAAGATGCGGCCGCTTAGCCGGACTGGCTGCCAAG-3' (reverse, NotI site is underlined) were designed to clone a full length human WWOX gene into the pENTRE vector (Invitrogen). Subsequently, WWOX in pENTRE vector was recombined into the destination vector pSLIK-Neo using the Gateway LR Clonase II Enzyme Mix (Invitrogen). To produce a lentivirus for establishing WWOX-inducible cells, pSLIK-Neo-WWOX and packing plasmids were transfected into HEK293FT packing cells using lipofectamine 2000. Lentiviruses were collected 72 h after transfection and used to transduce TSU-Pr1 and HCC1937 cells in a 6-well plate. The antibiotic G418 (800 µg/mL) was added to select drug-resistant cell populations 48 hours after transduction.
Quantitative PCR assay
Total RNAs were isolated using TRIzol (Invitrogen). Reverse transcriptions were performed using the Iscript cDNA synthesis kit (Bio-Rad). Quantitative RT-PCR was performed on an ABI-7300 system using RT2 SYBR Green reagents (Qiagen). Primers used for GAPDH and KLF5 (23) were the same as those previously published in our previous studies.
Cell viability assay
The cell viability was measured by Sulforhodamine B (SRB) assays (24). Briefly, TSU-Pr1 WWOX inducible cells were plated in 24-well plate at a density of 2×104/well. The day after plating, doxcycline was added to induce WWOX expression at a final concentration of 1 µg/mL (25). Cells were then fixed using 10% trichloroacetic acid (TCA) at the designed time, and stained with 0.4% SRB. After dissolving SRB from the available cells using 10 mM unbuffered Tris-base, optical results were read by an automated spectrophotometric plate reader at a single wavelength of 490-530 nm.
Immunoprecipitation (IP) and GST-pull down assays
The full length WWOX gene was cloned into the pcDNA3-3xMyc vector or pcDNA3-Flag vector using the pfu enzymes by PCR and expressed in HEK293T cells in order to detect the interaction between Flag-WWOX (or Myc-WWOX) and KLF5. WWOX constructs for GST-pull down experiments were obtained by subcloning WWOX fragments encoding full length or WW domain-deleted mutants into the BamHI and NotI sites of pEBG vector. The co-IP and GST pull-down experiments have been described in our previous studies (26,27).
Statistical analysis
Both the SRB and quantitative PCR assays were conducted in triplicate. Where appropriate, the resulting data were pooled to and expressed as means ± standard deviation and analyzed by t-test. P values less than 0.05 were considered to significant.
Results
WWOX suppresses KLF5 expression and cancer cell proliferation
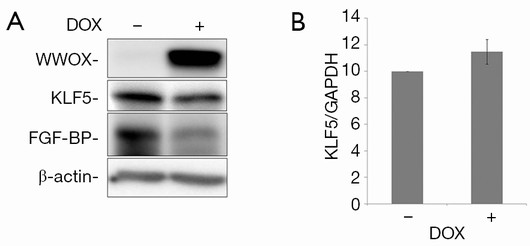
To test the effects of WWOX on KLF5, we established a Tet-on inducible WWOX expression system in TSU-Pr1 derived bladder cancer cell clone (K12) with stable KLF5 expression (28) and breast cancer cell line HCC1937. As shown in Figure 1, after doxycycline treatment, the expression of WWOX became significantly induced in TSU-Pr1 cells. We noted the induction of WWOX caused a significant decrease of the expression levels of KLF5 and KLF5’s well-characterized direct downstream target gene FGF-BP (13). Similarly, in breast cancer cell line HCC1937, WWOX induction also suppressed the expression of KLF5 and FGF-BP (Figure 2A). To test at what level WWOX regulates KLF5 expression, we performed quantitative PCR to detect the mRNA expression of KLF5. The induction of WWOX did not decrease KLF5 at the transcription level (Figure 2B), suggesting that WWOX may inhibits KLF5 at the post-transcription level, likely at the protein level. Subsequently, we then examined cell viability after WWOX induction and found that WWOX significantly suppressed cell proliferation as compared to the control in TSU-Pr1-K12 cells (Figure 1B).

WWOX interacts with KLF5
Since WWOX decreases the protein expression of KLF5, we were curious as to whether there was an interaction between WWOX and KLF5. We over-expressed Flag-WWOX and HA-Klf5 (mouse) in HEK293T cells. We observed that when Flag-WWOX was immunoprecipitated by anti-Flag antibody, the HA-Klf5 protein was detected (Figure 3A). These results suggested that WWOX and KLF5 proteins interact with one another, though the precise mechanisms were not clear.

WWOX typically interacts with its partners’ PY motifs through its WW domains. Since KLF5 contains a PY motif (26), we decided to test whether WWOX interacts with KLF5 through the WW domain-PY motif .We first tested if the PY motif was essential for the interaction and found that the wild type human KLF5 interacts with WWOX (Figure 3B), meanwhile KLF5 without the PY motif barely interacted with WWOX. Additionally, we demonstrated that GST-WWOX without both WW domains did not bind to KLF5 as compared to the wild type WWOX (Figure 3C). Interestingly, GST-WWOX without the first WW domain still interacted with KLF5 in the same manner as the wild type GST-WWOX, implicating the second WW domain of WWOX as being responsible for the binding to KLF5.
WWOX and KLF5 are reversely expressed in breast cells
In our previous studies, we observed that KLF5 is highly expressed in several basal-type breast cancer cell lines (for example, HCC1937) but lowly expressed in several luminal breast cancer cell lines (such as MCF7) (14). In the present study, we found a reverse expression pattern for WWOX and KLF5 (Figure 4). WWOX is highly expressed in two luminal breast cancer cell lines (MCF7, HCC1500) in which KLF5 is lowly expressed. In four normal breast cell lines (48-Pre, 184-Pre, MCF10A and 184B5) and two ER-PR- basal breast cancer cell lines (HCC1937 and HCC1806), the expression of WWOX was not detected and KLF5 was highly expressed. These results suggest that the expression of the WWOX protein seems inversely correlated with the expression of the KLF5 protein in breast epithelial cell lines.

Discussion
WWOX has been shown to function as a tumor suppressor in a number of cancers, including both breast and bladder cancer (1-3), but the underlying functional mechanisms of WWOX have not been fully explained. In the current study, we demonstrated that WWOX interacts with KLF5 and suppresses KLF5 protein expression at the posttranslational level. Our findings suggest that WWOX may suppress cancer cell proliferation, partially through down-regulating KLF5 expression because KLF5 is a pro-proliferative transcription factor.
We previously showed that KLF5 promotes cell proliferation by inducing FGF-BP expression (12,13). Here, we found that WWOX down-regulated the expression of KLF5 and FGF-BP (Figures 1,2). Likewise, induction of WWOX significantly suppressed KLF5-mediated cell proliferation. Taken together, it seems that WWOX suppresses the transcriptional activity and pro-proliferative function of KLF5 in cancer cells.
The KLF5 protein is tightly regulated by the ubiquitin-proteasome pathway (26). Several WW-domain containing E3 ligases, such as WWP1 and Smurf2, were shown to target KLF5 for ubiquitin-mediated degradation (26,29). In a recent study, we showed that two WW domain containing proteins, YAP and TAZ, interact with KLF5 and blocked WWP1-mediated KLF5 degradation (30,31). WWOX did not regulate KLF5 at the mRNA level (Figure 2) and WWOX interacts with KLF5 through WW-domain and PY motif interaction (Figure 3). It is possible that WWOX recruits other E3 ubiquitin ligases to target KLF5 for ubiquitin-proteasome degradation, which might also be responsible for the reverse expression of WWOX and KLF5 proteins. Though these findings mark some interesting and potentially novel understandings on the relationship between WWOX and KLF5, the precise mechanism by which WWOX decreases the KLF5 protein expression need further investigation.
KLF5 has been reported to be an independent prognosis biomarker in breast cancer because patients with higher KLF5 mRNA expression tend to have a shorter survival than those with lower KLF5 expression (10). Similarly, KLF5 protein levels are higher in basal-like invasive breast cancer cells than those in non-basal-like cancer cells (14,16). In the present study, we found that the WWOX expression is much lower or even undetectable in basal type breast cancer cells (Figure 4). This finding is consistent with observations of patient samples (7,21) indicate that both WWOX and KLF5 may serve as potential molecular biomarkers for basal-type invasive breast cancers. Future studies would be investigating the protein expression of WWOX/KLF5 in a larger number of clinical breast tumor samples by IHC, which may provide clinical support of our preliminary experimental findings.
Conclusions
In summary, we demonstrated that WWOX suppresses the expression of KLF5 and its target gene FGF-BP at the protein level as well as suppressing cancer cell proliferation. We also found that WWOX interacts with KLF5 via WW domain-PY motif. Interestingly, the expression of WWOX was negatively correlated with that of KLF5 in a panel of breast cancer cell lines. These findings may provide rationale for developing WWOX and KLF5 as breast cancer diagnosis or prognosis biomarkers.
Acknowledgements
We would like to thank Dr. Vincent W. Yang from the Emory University School of Medicine for kindly providing the mouse HA-Klf5 construct, as well as Dr. Kevin Pumiglia from Albany Medical Center for providing empty vectors for generating WWOX inducible cell lines. This study was supported by National Natural Science Foundation of China (81272930, 81322038, 31260208, and U1132605), the Science and Technological Key Project of Yunnan Province (2012FB185) and West Light Foundation of the Chinese Academy of Sciences (to R.L.).
Disclosure: The authors declare no conflict of interest.
References
- Del Mare S, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem 2009;108:737-45. [PubMed]
- Salah Z, Aqeilan R, Huebner K. WWOX gene and gene product: tumor suppression through specific protein interactions. Future Oncol 2010;6:249-59. [PubMed]
- Iliopoulos D, Guler G, Han SY, et al. Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene 2005;24:1625-33. [PubMed]
- Aqeilan RI, Trapasso F, Hussain S, et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci U S A 2007;104:3949-54. [PubMed]
- Bednarek AK, Laflin KJ, Daniel RL, et al. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res 2000;60:2140-5. [PubMed]
- Aqeilan RI, Pekarsky Y, Herrero JJ, et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci U S A 2004;101:4401-6. [PubMed]
- Guler G, Huebner K, Himmetoglu C, et al. Fragile histidine triad protein, WW domain-containing oxidoreductase protein Wwox, and activator protein 2gamma expression levels correlate with basal phenotype in breast cancer. Cancer 2009;115:899-908. [PubMed]
- Aqeilan RI, Donati V, Gaudio E, et al. Association of Wwox with ErbB4 in breast cancer. Cancer Res 2007;67:9330-6. [PubMed]
- Aqeilan RI, Donati V, Palamarchuk A, et al. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res 2005;65:6764-72. [PubMed]
- Tong D, Czerwenka K, Heinze G, et al. Expression of KLF5 is a prognostic factor for disease-free survival and overall survival in patients with breast cancer. Clin Cancer Res 2006;12:2442-8. [PubMed]
- Chen C, Benjamin MS, Sun X, et al. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int J Cancer 2006;118:1346-55. [PubMed]
- Liu R, Zheng HQ, Zhou Z, et al. KLF5 promotes breast cell survival partially through fibroblast growth factor-binding protein 1-pERK-mediated dual specificity MKP-1 protein phosphorylation and stabilization. J Biol Chem 2009;284:16791-8. [PubMed]
- Zheng HQ, Zhou Z, Huang J, et al. Krüppel-like factor 5 promotes breast cell proliferation partially through upregulating the transcription of fibroblast growth factor binding protein 1. Oncogene 2009;28:3702-13. [PubMed]
- Liu R, Zhou Z, Zhao D, et al. The induction of KLF5 transcription factor by progesterone contributes to progesterone-induced breast cancer cell proliferation and dedifferentiation. Mol Endocrinol 2011;25:1137-44. [PubMed]
- Yang Y, Tetreault MP, Yermolina YA, et al. Krüppel-like factor 5 controls keratinocyte migration via the integrin-linked kinase. J Biol Chem 2008;283:18812-20. [PubMed]
- Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008;40:499-507. [PubMed]
- Desmedt C, Ruíz-García E, André F. Gene expression predictors in breast cancer: current status, limitations and perspectives. Eur J Cancer 2008;44:2714-20. [PubMed]
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [PubMed]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [PubMed]
- Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418-23. [PubMed]
- Wang X, Chao L, Ma G, et al. The prognostic significance of WWOX expression in patients with breast cancer and its association with the basal-like phenotype. J Cancer Res Clin Oncol 2011;137:271-8. [PubMed]
- Chen C, Sun X, Ran Q, et al. Ubiquitin-proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene 2005;24:3319-27. [PubMed]
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33. [PubMed]
- Chen C, Zhou Z, Ross JS, et al. The amplified WWP1 gene is a potential molecular target in breast cancer. Int J Cancer 2007;121:80-7. [PubMed]
- Shin KJ, Wall EA, Zavzavadjian JR, et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci U S A 2006;103:13759-64. [PubMed]
- Chen C, Sun X, Guo P, et al. Human Kruppel-like factor 5 is a target of the E3 ubiquitin ligase WWP1 for proteolysis in epithelial cells. J Biol Chem 2005;280:41553-61. [PubMed]
- Li Y, Zhou Z, Chen C. WW domain-containing E3 ubiquitin protein ligase 1 targets p63 transcription factor for ubiquitin-mediated proteasomal degradation and regulates apoptosis. Cell Death Differ 2008;15:1941-51. [PubMed]
- Chen C, Benjamin MS, Sun X, et al. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int J Cancer 2006;118:1346-55. [PubMed]
- Du JX, Hagos EG, Nandan MO, et al. The E3 ubiquitin ligase SMAD ubiquitination regulatory factor 2 negatively regulates Krüppel-like factor 5 protein. J Biol Chem 2011;286:40354-64. [PubMed]
- Zhi X, Zhao D, Zhou Z, et al. YAP promotes breast cell proliferation and survival partially through stabilizing the KLF5 transcription factor. Am J Pathol 2012;180:2452-61. [PubMed]
- Zhao D, Zhi X, Zhou Z, et al. TAZ antagonizes the WWP1-mediated KLF5 degradation and promotes breast cell proliferation and tumorigenesis. Carcinogenesis 2012;33:59-67. [PubMed]

