Annual cost of illness of stomach and esophageal cancer patients in urban and rural areas in China: A multi-center study
Introduction
Stomach cancer and esophageal cancer are huge threats to the health of Chinese people. According to International Agency for Research on Cancer (IARC), in 2012, 45% of global stomach cancer cases and 47% of global deaths occurred in China, the percentages for esophageal cancer were 50% and 50%, respectively (1). Such proportions were astonishingly high given that Chinese population was only one fifth of that of the world. Both cancers were among the most diagnosed cancers in China (2). It is estimated that 427,100 new stomach cancer cases and 301,200 deaths, and 276,900 new esophageal cancer cases and 206,500 deaths occurred in 2013 nationwide (3).
Compared with the abundant evidence on the epidemiological burden of stomach and esophageal cancer, only a few researches had been done concerning the economic burden of them at patient level in China (4-6). Moreover, these studies were carried out in either one area (county, province) (5,6) or only urban areas (4). To date, in-depth studies on the cost of illness of stomach or esophageal cancer at patient level have been conducted mainly in developed countries (7-12). In the patients who survived beyond 1 year in Ontario, esophageal cancer was the costliest cancer while stomach cancer was the fifth costliest (9). Australian and British researchers unveiled that surgery and stage I were associated with higher hospitalization costs of esophageal cancer (7,8), whereas a Korean study showed that advanced stages were associated with higher total costs (10). Furthermore, age was found to be positively associated with the hospitalization cost of stomach cancer by Japanese researchers (11).
In 2009, medical expenses due to stomach cancer treatment accounted for 10% of that of all cancers in Japan. A downward trend had been noticed nevertheless and was projected to continue in the near future (13). The payments of cancer inpatients in China, unlike Japan, had increased by 84.1% from 2011 to 2015, reaching a total of 28.4 billion US dollars. Of which, stomach and esophageal cancer accounted for approximately 2.0 and 1.2 billion respectively (14). Therefore, it is of vital importance to further investigate this tremendous financial burden of stomach and esophageal cancer in this country.
China was among the countries with the largest gains of Healthcare Quality and Access Index during 1990−2015 (15), thanks to the rapid economic development and health care reform (16). In 2009, Central Government of China launched a $125 billion medical reform, aiming at achieving comprehensive universal health coverage by 2020 (17). To better understand the financial burden of Chinese patients with stomach and esophageal cancer, provide information for the amendment of the policy on cancer prevention and control, direct the medical insurance to a more efficient way, and consequently support the implementation of medical reform and enhance the quality of health service for the Chinese population, herein we provided an in-depth description of the annual cost of illness (ACI) of stomach cancer and esophageal cancer patients in China, using the data collected in seven cities/counties, where we carried out a randomized controlled trial (RCT) to evaluate the efficacy of screening for upper gastrointestinal cancer (18). Comparisons of the ACI were made as well, especially between urban and rural areas.
Materials and methods
Data sources and collection
Seven cities/counties, which are Harbin, Changsha, Linzhou, Cixian, Wuwei, Sheyang and Luoshan, were selected as study sites, covering both urban and rural areas in China with a vast geographical span as shown in Figure 1. A detailed description of the selecting criteria of the study sites can be found in our previous article (18). The hospitals selected to participate in this study were either the largest or the only cancer hospital or general hospital in each city/county, being in charge of the medical service for the majority of the local residents. Altogether seven hospitals were selected, each in one of the seven study sites.
To obtain the cost per hospitalization (CPH), data of all patients discharged between 1st September 2015 and 31st August 2016 were extracted from the electronic medical record of the seven hospitals, with the primary discharge diagnosis being stomach cancer or esophageal cancer. Patients with both cancers were excluded. Besides demographic information, extracted data included the medical record number, ID number, date of admission, date of discharge, length of stay (LOS), clinical stage, pathologic type, primary treatment, total hospitalization cost and itemized hospitalization cost of each case. If any information happened to be missing or illogical, staff in the study sites would check manually in the paper medical record. Modification or deletion would be made according to the feedback.
To obtain the annual number of hospitalization, annual direct non-medical cost and annual indirect cost, we sampled the former patients of the seven hospitals discharged before 1st September 2015 diagnosed as stomach cancer or esophageal cancer. Patients with both cancers were excluded. We chose approximately 150 patients for each cancer in each area. Balance of sex and clinical stage were considered in the selection to ensure that the numbers of stage I to IV patients were not less than 30 in each area. Patients were selected backwardly based on the discharge date in the electronic medical record until the expected sample size was reached. Besides demographic information, we also collected ID number, discharge diagnosis, LOS, clinical stage, pathologic type, primary treatment, the number of hospitalization in the past year, direct non-medical cost (nutraceutical fee and commission to caregivers) and indirect cost (productivity loss of the patients and their family members) in the past year.
Statistical analysis
All treatments were classified into five modalities: surgery, radiotherapy, chemotherapy, concurrent chemoradiotherapy and palliative care. Pathologic types were classified into adenocarcinoma and other for stomach cancer, squamous cell carcinoma, adenocarcinoma and other for esophageal cancer, according to the distribution of all pathologic types of each cancer in Chinese population (19,20). Patients were also categorized as elderly group (≥60 years old) and non-elderly group (<60 years old). The hospitalization cost was itemized into eight parts: western medicine fee, diagnosis fee, material fee, surgery fee, non-surgical treatment fee, traditional Chinese medicine fee, ward fee and others. The proportion of each part in the total CPH was calculated.
Annual direct medical cost was calculated by multiplying the CPH extracted from the electronic medical record by the number of hospitalization of the sampled patients. ACI was calculated by adding the average values of annual direct medical cost, direct non-medical cost and indirect cost together. All costs were converted to 2016 US dollars (1 USD=6.6423 RMB).
After logarithm transition, two-sample Student’s t-test was used for binary classification variables, and ANOVA test was used for other multiple categorical variables to compare the difference of CPH. All statistical tests were two-sided. P<0.05 was considered statistically significant. All analyses were carried out using STATA (Version 14.2; StataCorp LLC, TX, USA).
Results
Distribution of patient-level characteristics
As shown in Table 1, altogether 19,986 cases (13,528 with stomach cancer and 6,458 with esophageal cancer) were included in the analysis of CPH, with a mean age of 58.5 and 63.0 years for stomach and esophageal cancer patients respectively. Male patients were the majority by far. In all seven study sites, stage III and stage II were the most reported for stomach cancer and esophageal cancer respectively. The most prevalent pathologic type was adenocarcinoma (87.81%) for stomach cancer and squamous cell carcinoma (88.81%) for esophageal cancer. Chemotherapy was the most adopted treatment for both cancers. The mean LOS was 9.3 d for stomach cancer and 16.9 d for esophageal cancer while the median LOS was 7 d and 10 d, respectively.

Full table
Patients were younger in urban areas than in rural areas for both cancers. Stage II was the most reported in rural esophageal cancer patients while stage III was the most reported in other subgroups. Mean LOS were longer in rural areas than in urban areas for both cancers. Chemotherapy was the most adopted treatment for stomach cancer patients in both areas as well as esophageal cancer patients in urban areas, while in rural areas radiotherapy was the most adopted for esophageal cancer patients. Distribution of demographic and clinical characteristics of former patients included in the analysis of numbers of hospitalization, annual direct non-medical cost and annual indirect cost could be found in Supplementary Table S1.

Full table
ACI of stomach and esophageal cancer patients
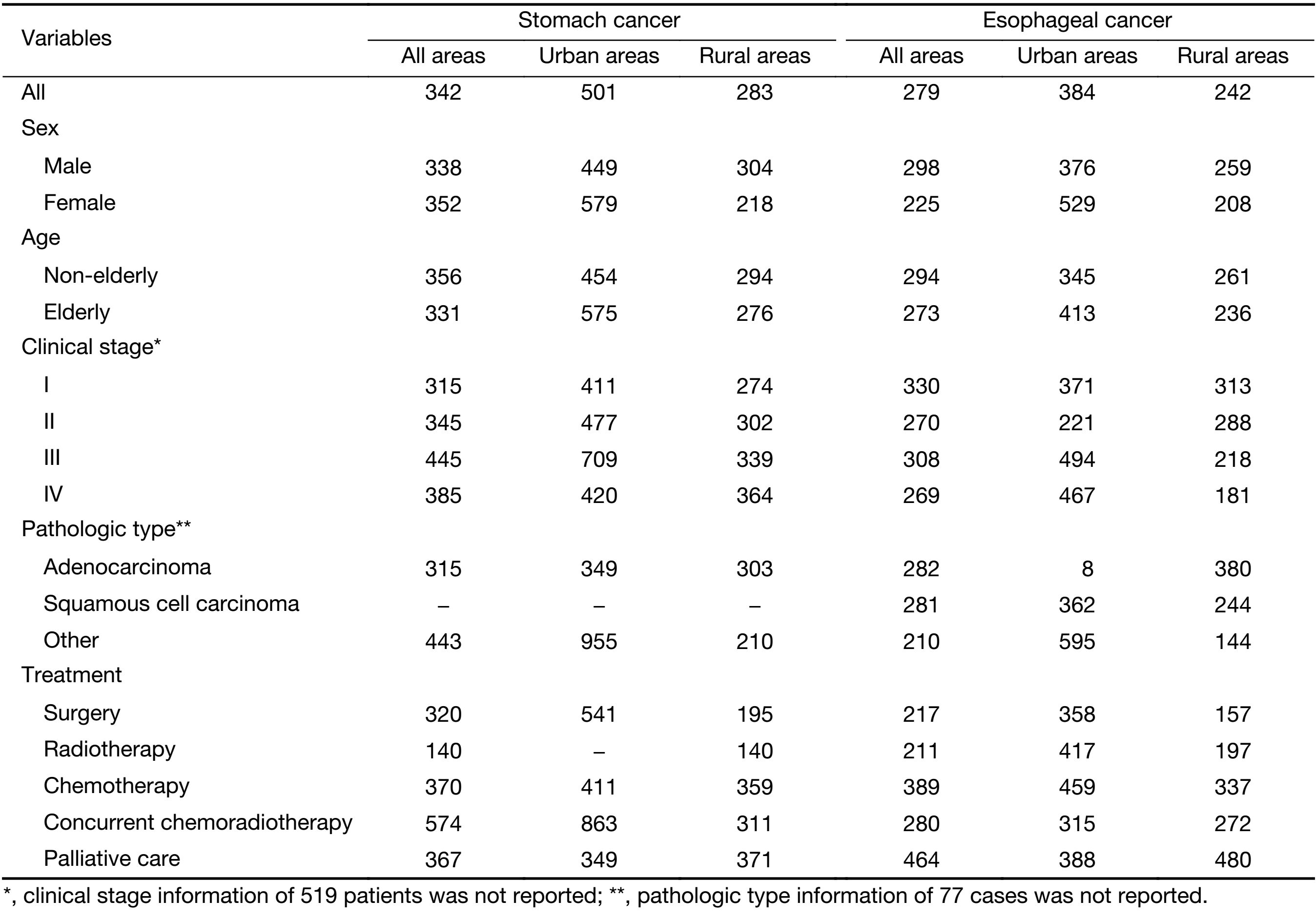
As shown in Table 2, ACI of stomach and esophageal cancer patients in all seven study sites were $5,694 and $6,342, respectively. Non-elderly patients generally had greater ACI compared with the elderly ones for both cancers. ACI of adenocarcinoma was the greatest for both cancers. Furthermore, stage IV was associated with the lowest ACI for both cancers in all stages while surgery was associated with the highest in all the treatment modalities, followed by concurrent chemoradiotherapy, chemotherapy, palliative care and radiotherapy.

Full table
ACI in urban areas were $10,449 for stomach cancer and $13,029 for esophageal cancer while the values were $2,927 and $3,504 respectively in rural areas. For both cancers, ACI of men was greater than that of women in both areas. The financial burden associated with early stages (I and II) were substantially higher than that associated with advanced stages (III and IV) in urban areas, while no such difference was found in rural areas. Surgery was associated with the highest ACI in urban areas while it was preceded by concurrent chemoradiotherapy in rural areas for both cancers.
CPH of stomach and esophageal cancer patients
As shown in Table 3, CPH of stomach and esophageal cancer patients in all seven study sites were $2,294 and $2,863 respectively. In urban areas, elderly patients were associated with greater CPH, while in rural areas, a negative association between age and CPH was found. Furthermore, differences of CPH among clinical stages and treatment modalities were statistically significant for both cancers (P<0.0001), with stage I and surgery having the greatest CPH.

Full table
CPH in urban areas were $2,938 for stomach cancer and $3,994 for esophageal cancer while the values were $1,224 and $1,768, respectively in rural areas. Unlike the patterns shown in all areas, no significant sexual difference of CPH was found while greater CPH was constantly associated with elderly patients for both cancers in urban and rural areas. Significant difference of CPH among clinical stages was found for both cancers only in urban areas and early stages were generally associated with greater CPH. Except for rural esophageal cancer patients, surgery had the highest CPH among all treatment modalities.
Proportional breakdown of CPH
As shown in Figure 2, for stomach cancer, western medicine fee accounted for the largest proportion of CPH in both areas (urban: 42.7%, rural: 43.0%), followed by material fee (25.2%) and diagnosis fee (15.3%) in urban areas, diagnosis fee (22.9%) and non-surgical treatment fee (11.2%) in rural areas. For esophageal cancer, western medicine fee had the largest proportion in both areas as well (urban: 37.8%, rural: 34.3%), followed by material fee (19.8%) and non-surgical treatment fee (17.2%) in urban areas, non-surgical treatment fee (34.0%) and diagnosis fee (16.6%) in rural areas.
Discussion
In this study, we examined the ACI of stomach and esophageal cancer patients in urban and rural areas in China using year-long data from seven cities/counties. We found that ACI of urban stomach and esophageal cancer patients were $10,449 and $13,029 while ACI of rural patients were $2,927 and $3,504, respectively. By contrast, in 2016, annual income of urban and rural residents in China were $5,061 and $1,861, respectively (21). Male and non-elderly patients were associated with heavier burden while other indicators of heavy burden were early stages and surgery as the primary treatment modality. Annual direct medical costs, direct non-medical costs and indirect costs were shown in Supplementary Table S2, S3 and S4.

Full table
Full table

Full table
In our study, the financial burden of esophageal cancer was heavier compared with stomach cancer, as proved by previous studies in Ontario (9) and Hua county, a high-risk area of upper gastrointestinal cancer (5), whereas in Anhui province, a typical inland province in China, stomach cancer was the costliest cancer (6), The proportion of men was substantially higher than that of women in our analysis, as was found of the incidence rate in population-based cancer registration (3,20).
Urban costs were constantly higher than rural costs in our study, partly because urban data were extracted from two Grade 3 hospitals, which are both provincial hospitals, while rural data were mainly from Grade 2 and county level hospitals. Hospitals in China were categorized into three grades based on their function and facilities, with Grade 3 as the highest. Previous studies pointed out that medical cost increased monotonically with hospital level and grade (5,22). The gap of costs indicated that the lopsided distribution of health resources between urban and rural China still existed (23) since hospitals with high levels are more likely to be located in cities rather than counties.
Previous studies in China tended to categorize age into four or more groups and found no clear association between medical expenditure and age group (4,6). In this study, we categorized age into two groups and found that for both cancers, non-elderly patients were associated with lower CPH and more frequent hospitalization (Supplementary Table S5) in urban and rural areas whereas in Japan, greater medical costs were associated with elderly patients (11). As a result, heavier ACI was found for non-elderly patients in all areas combined, as was found in a study on colorectal, breast and prostate cancer patients in US (24). Though evidences showed that age did not affect overall surgical outcomes (25,26), surgery was still applied less frequently to elderly patients (27). Overall mean cost of surgical group was higher than that of non-surgical group, according to an Australian study (8), further proved our results. One main reason for the high cost of surgery was the postoperative complication (28,29). Additional costs of severe complications could account for 27% of the total hospitalization costs (28). Hospitalization costs reduction could be achieved by not only reducing complications after surgery but also increasing the number of experienced surgeons (29,30). Moreover, high costs notwithstanding, surgical resection has the greatest benefit in terms of survival and is proved to be at least as cost-effective as other treatment modalities (31).

Full table
ACI generally decreased as the clinical stage increased for both cancers, especially in urban areas. Guo et al. (4) reported that for esophageal cancer, stage II patients had the highest medical expenditure during 2009−2011 in urban China. In terms of hospitalization cost per patient that covered the first year after diagnosis, stage II was the most expensive for esophageal cancer patients in Northern Ireland as well (7) whereas in Korea, advanced stages at diagnosis were associated with 1.8−2.5 folds higher costs (10). Compared with patients in US, Chinese stomach cancer patients had larger tumors and later stages (32), which were generally associated with shorter survival (33). The majority of patients included in this study were also at stage III or IV. Therefore, even though screening could detect cancer at early stage and was proved cost-effective or cost-benefit by Chinese and international studies (34-36), the possibility that the annual expenditures on treatment may increase along with survival remains to the beneficiaries of screening programs.
Urban employee basic medical insurance (UEBMI), urban resident basic medical insurance (URBMI) and new rural cooperative medical scheme (NCMS) are the three mainstream health insurance schemes in China. By 2010, the percentage of the Chinese population covered by these three schemes rose from 23% in 2003 to over 90% (37). Notwithstanding, the financial protections offered are very modest and out-of-pocket spending was hardly reduced (37,38). The findings in this study quantified the ACI of stomach and esophageal cancer of Chinese patients in both urban and rural areas, suggesting that preferential medical insurance policies should be designed at least in the high-risk areas of upper gastrointestinal cancer to further reduce the health inequalities (38).
Our research had the following strengths. The seven chosen hospitals are responsible for the treatment of the majority of the local residents in the seven study sites which cover both urban and rural areas and three economical-geographical regions in China (18). Data of all cases discharged in the whole year were extracted without omission and the results of urban and rural areas were reported separately.
The results of our study should be seen in the light of its limitations as well. The five types of therapy in this research are all broad concepts containing various methods whereas the LOS and cost of each method varied (39-43). In this research, all methods of each type of therapy were classified simply into one modality. Consequently, the inherent difference between each method could not be analyzed. Therefore, further investigations with more elaborate designs were needed.
Conclusions
Stomach cancer and esophageal cancer are imposing tremendous burdens on Chinese people both epidemiologically and financially. We observed a huge gap of the ACI of stomach and esophageal cancer between urban and rural areas, indicating the lopsided distribution of health resources in China and the burden of esophageal cancer was constantly heavier than stomach cancer. Furthermore, the burden varied substantially with clinical stage, treatment modality, pathologic type and other clinical characteristics. Preferential policies of medical insurance should be designed to tackle with this burden, especially in the high-risk areas of stomach or esophageal cancer.
Acknowledgements
We acknowledged the cooperation of all the staff in the seven study sites for understanding our concept and collecting the data. This study was supported by the Special Fund for Health Research in the Public Interest (No. 201502001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ervik M, Ferlay J, Mery L, et al. Cancer Today. International Agency for Research on Cancer, Lyon, France. 2016. Available online: http://gco.iarc.fr/today.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [PubMed] DOI:10.3322/caac.21338
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett 2017;401:63–71. [PubMed] DOI:10.1016/j.canlet.2017.04.024
- Guo LW, Huang HY, Shi JF, et al. Medical expenditure for esophageal cancer in China: a 10-year multicenter retrospective survey (2002-2011). Chin J Cancer 2017;36:73. [PubMed] DOI:10.1186/s40880-017-0242-3
- Li X, Cai H, Wang C, et al. Economic burden of gastrointestinal cancer under the protection of the New Rural Cooperative Medical Scheme in a region of rural China with high incidence of oesophageal cancer: cross-sectional survey. Trop Med Int Health 2016;21:907–16. [PubMed] DOI:10.1111/tmi.12715
- Zhao T, Cheng J, Chai J, et al. Inpatient care burden due to cancers in Anhui, China: a cross-sectional household survey. BMC Public Health 2016;16:308. [PubMed] DOI:10.1186/s12889-016-2995-z
- Agus AM, Kinnear H, O’Neill C, et al. Description and predictors of hospital costs of oesophageal cancer during the first year following diagnosis in Northern Ireland. Eur J Cancer Care (Engl) 2013;22:450–8. [PubMed] DOI:10.1111/ecc.12046
- Gordon LG, Eckermann S, Hirst NG, et al. Healthcare resource use and medical costs for the management of oesophageal cancer. Br J Surg 2011;98:1589–98. [PubMed]
- de Oliveira C, Bremner KE, Pataky R, et al. Understanding the costs of cancer care before and after diagnosis for the 21 most common cancers in Ontario: a population-based descriptive study. CMAJ Open 2013;1:E1–8. [PubMed] DOI:10.9778/cmajo.20120013
- Shin JY, Kim SY, Lee KS, et al. Costs during the first five years following cancer diagnosis in Korea. Asian Pac J Cancer Prev 2012;13:3767–72. [PubMed]
- Murata A, Muramatsu K, Ichimiya Y, et al. Endoscopic submucosal dissection for gastric cancer in elderly Japanese patients: an observational study of financial costs of treatment based on a national administrative database. J Dig Dis 2014;15:62–70. [PubMed] DOI:10.1111/1751-2980.12106
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117–28. [PubMed] DOI:10.1093/jnci/djq495
- Haga K, Matsumoto K, Kitazawa T, et al. Cost of illness of the stomach cancer in Japan — a time trend and future projections. BMC Health Serv Res 2013;13:283. [PubMed] DOI:10.1186/1472-6963-13-283
- Cai Y, Xue M, Chen W, et al. Expenditure of hospital care on cancer in China, from 2011 to 2015. Chin J Cancer Res 2017;29:253–62. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.03.11
- GBD 2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990-2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet 2017;390:231–66. [PubMed] DOI:10.1016/S0140-6736(17)30818-8
- Chen Z. Launch of the health-care reform plan in China. Lancet 2009;373:1322–4. [PubMed] DOI:10.1016/S0140-6736(09)60753-4
- Yip WC, Hsiao WC, Chen W, et al. Early appraisal of China’s huge and complex health-care reforms. Lancet 2012;379:833–42. [PubMed] DOI:10.1016/S0140-6736(11)61880-1
- Chen W, Zeng H, Chen R, et al. Evaluating efficacy of screening for upper gastrointestinal cancer in China: a study protocol for a randomized controlled trial. Chin J Cancer Res 2017;29:294–302. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.04.02
- He J, Chen W. Chinese cancer registry annual report, 2016. Beijing: Tsinghua University Press, 2017.
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [PubMed] DOI:10.3322/caac.21262
- Department of Household Surveys, National Bureau of Statistics of China. China Household Survey, 2017. Beijing: National Bureau of Statistics of China, 2017. Available online: http://www.stats.gov.cn/ztjc/zdtjgz/yblh/zysj/201710/t20171010_1540707.html
- Li R, Zhang L, Yang J, et al. Analysis of inpatient payments of breast cancer patients with different medical insurance coverages in China (mainland) in 2011-2015. Chin J Cancer Res 2017;29:419–25. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.05.06
- Liu Y, Rao K, Wu J, et al. China’s health system performance. Lancet 2008;372:1914–23. [PubMed] DOI:10.1016/S0140-6736(08)61362-8
- Zheng Z, Yabroff KR, Guy GP Jr., et al.. Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. J Natl Cancer Inst 2015;108. DOI:10.1093/jnci/djv382
- McLoughlin JM, Lewis JM, Meredith KL. The impact of age on morbidity and mortality following esophagectomy for esophageal cancer. Cancer Control 2013;20:144–50. [PubMed] DOI:10.1177/107327481302000208
- O’Grady G, Hameed AM, Pang TC, et al. Patient selection for oesophagectomy: impact of age and comorbidities on outcome. World J Surg 2015;39:1994–9. [PubMed] DOI:10.1007/s00268-015-3072-y
- Nienhueser H, Kunzmann R, Sisic L, et al. Surgery of gastric cancer and esophageal cancer: Does age matter?. J Surg Oncol 2015;112:387–95. [PubMed] DOI:10.1002/jso.24004
- Goense L, van Dijk WA, Govaert JA, et al. Hospital costs of complications after esophagectomy for cancer. Eur J Surg Oncol 2017;43:696–702. [PubMed] DOI:10.1016/j.ejso.2016.11.013
- Selby LV, Gennarelli RL, Schnorr GC, et al. Association of hospital costs with complications following total gastrectomy for gastric adenocarcinoma. JAMA Surg 2017;152:953–8. [PubMed] DOI:10.1001/jamasurg.2017.1718
- Murata A, Okamoto K, Muramatsu K, et al. Time trend of medical economic outcomes of endoscopic submucosal dissection for gastric cancer in Japan: a national database analysis. Gastric Cancer 2014;17:294–301. [PubMed] DOI:10.1007/s10120-013-0282-6
- Farndon MA, Wayman J, Clague MB, et al. Cost-effectiveness in the management of patients with oesophageal cancer. Br J Surg 1998;85:1394–8. [PubMed] DOI:10.1046/j.1365-2168.1998.00916.x
- Strong VE, Wu AW, Selby LV, et al. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol 2015;112:31–7. [PubMed] DOI:10.1002/jso.23940
- Rouvelas I, Zeng W, Lindblad M, et al. Survival after surgery for oesophageal cancer: a population-based study. Lancet Oncol 2005;6:864–70. [PubMed] DOI:10.1016/S1470-2045(05)70347-8
- Wei WQ, Yang CX, Lu SH, et al. Cost-benefit analysis of screening for esophageal and gastric cardiac cancer. Chin J Cancer 2011;30:213–8. [PubMed] DOI:10.5732/cjc.010.10425
- Abd Elrazek AE, Eid KA, El-Sherif AE, et al. Screening esophagus during routine ultrasound: medical and cost benefits. Eur J Gastroenterol Hepatol 2015;27:8–12. [PubMed] DOI:10.1097/MEG.0000000000000196
- Yeh JM, Hur C, Ward Z, et al. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: a cost-effectiveness analysis. Gut 2016;65:563–74. [PubMed] DOI:10.1136/gutjnl-2014-308588
- Tang S, Brixi H, Bekedam H. Advancing universal coverage of healthcare in China: translating political will into policy and practice. Int J Health Plann Manage 2014;29:160–74. [PubMed] DOI:10.1002/hpm.2207
- Cheng L, Liu H, Zhang Y, et al. The impact of health insurance on health outcomes and spending of the elderly: evidence from China’s New Cooperative Medical Scheme. Health Econ 2015;24:672–91. [PubMed] DOI:10.1002/hec.3053
- Shinohara T, Kawano S, Tanaka Y, et al. Comparison of the cost and outcomes following totally laparoscopic and laparoscopy-assisted distal gastrectomies for gastric cancer: a single-institution comparison. Surg Endosc 2016;30:3573–81. [PubMed] DOI:10.1007/s00464-015-4656-1
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015;9:174–80. [PubMed] DOI:10.5009/gnl13299
- Hoya Y, Taki T, Tanaka Y, et al. Disadvantage of operation cost in laparoscopy-assisted distal gastrectomy under the national health insurance system in Japan. Dig Surg 2010;27:343–6. [PubMed] DOI:10.1159/000318774
- He J, Wen F, Yin X, et al. Cost analysis of S1 and XELOX as adjuvant therapy for gastric cancer. Anticancer Drugs 2013;24:754–8. [PubMed] DOI:10.1097/CAD.0b013e328361bef2
- Zhou KR, Cheng A, Ng WT, et al. Cost minimization analysis of capecitabine versus 5-fluorouracil-based treatment for gastric cancer patients in Hong Kong. J Med Econ 2017;20:541–8. [PubMed] DOI:10.1080/13696998.2017.1296452



